This article is about Electrochemical Cell: Unraveling the Power of Redox Reactions.
In the world of energy conversion and storage, electrochemical cells stand as remarkable creations that bridge the gap between chemistry and electricity. From the batteries that power our devices to the fundamental processes driving biological systems, electrochemical cells play a pivotal role. This article will deeply explain into the intricate workings of electrochemical cells, exploring their types, components, mechanisms, and the diverse array of applications that harness their potential.
Understanding Electrochemical Cells
At its core, an electrochemical cell is a device that converts chemical energy directly into electrical energy, or vice versa. This process hinges on the principle of redox reactions, which involve the transfer of electrons between chemical species. The term “redox” is derived from “reduction” and “oxidation,” where reduction refers to gaining electrons, and oxidation involves losing electrons. Electrochemical cells consist of two distinct compartments, known as half-cells, each containing a conductor immersed in an electrolyte solution.
Components of Electrochemical Cells
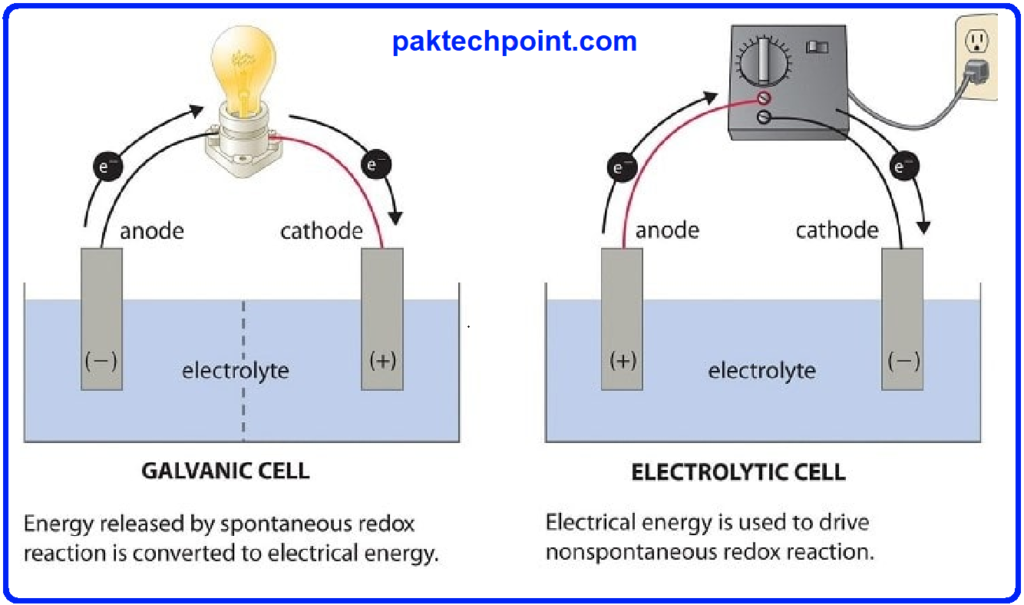
- Electrodes: Electrodes serve as conductive interfaces between the electrolyte and the external circuit. The anode is where oxidation occurs, releasing electrons into the circuit, while the cathode is the site of reduction, where electrons are accepted from the circuit.
- Electrolyte: The electrolyte is a solution containing ions that facilitate the flow of charge between the two half-cells. It enables the movement of ions required to maintain electrical neutrality during the redox reaction.
- Separator: The separator physically separates the two half-cells while allowing ion flow between them. It prevents direct contact between the electrodes, preventing short circuits.
- External Circuit: The external circuit connects the anode and cathode, allowing the flow of electrons generated by the redox reaction. This flow of electrons constitutes electrical current.
Types of Electrochemical Cells
Electrochemical cells can be broadly categorized into two types based on their operational mode: galvanic (voltaic) cells and electrolytic cells.
- Galvanic Cells: Galvanic cells, commonly known as voltaic cells, convert spontaneous chemical reactions into electrical energy. The most familiar example is the common battery, where chemical reactions within the cells generate a potential difference, leading to the flow of electrons and the production of electric current.
- Electrolytic Cells: In contrast, electrolytic cells operate by utilizing electrical energy to drive non-spontaneous reactions. These cells are employed in processes like electroplating and metal refining, where the application of an external voltage causes the desired redox reactions to occur.
Mechanism and Working of Electrochemical Cells
The operation of an electrochemical cell is a dance of electrons and ions orchestrated by redox reactions. At the anode, oxidation results in the release of electrons into the external circuit, leaving behind positively charged ions in the electrolyte. These electrons travel through the circuit to the cathode, where reduction takes place, and electrons are accepted along with positive ions from the electrolyte. This flow of electrons constitutes the electric current, which powers devices or drives non-spontaneous reactions.
Applications of Electrochemical Cells
- Batteries: Electrochemical cells power a multitude of devices, from the humble flashlight to cutting-edge electric vehicles. Lithium-ion batteries, commonly found in portable electronics, smartphones, and electric cars, rely on the redox reactions within electrochemical cells to store and deliver energy efficiently.
- Fuel Cells: Fuel cells employ electrochemical cells to generate electricity through the continuous oxidation of fuel (like hydrogen) and reduction of an oxidizing agent (like oxygen). This technology holds the promise of clean energy conversion, producing electricity and water as the only byproducts.
- Electrolysis: Electrolytic cells find use in various industrial processes, including metal electroplating, water electrolysis for hydrogen production, and the extraction of metals from their ores. These applications harness the power of electrochemical cells to achieve precise and controlled chemical transformations.
- Biological Systems: Electrochemical cells are at the heart of many biological processes. Neurons in our brains use ion gradients created by these cells to transmit electrical signals, enabling cognition and movement.
The Fusion of Chemistry and Electricity
The realm of electrochemical cells unites the fundamental principles of chemistry and electricity, yielding a diverse array of applications that power our world and shape our technological landscape. These cells embody the elegance of redox reactions and their capacity to transform energy in a myriad of ways, from the compact batteries in our pockets to the monumental fuel cells that could redefine our energy future. As research and innovation continue to push the boundaries of materials and design, electrochemical cells stand as an enduring testament to human ingenuity and the fascinating interplay of science and engineering.