Cathodic Protection (CP) is a technique used to prevent the corrosion of metal surfaces by making the metal the cathode of an electrochemical cell. It is widely employed to protect various metal structures, such as pipelines, tanks, and bridges, from the corrosive effects of the surrounding environment. The primary objective of a cathodic protection system is to reduce the corrosion rate of the protected metal surface, thereby extending the structure’s lifespan and ensuring its integrity.
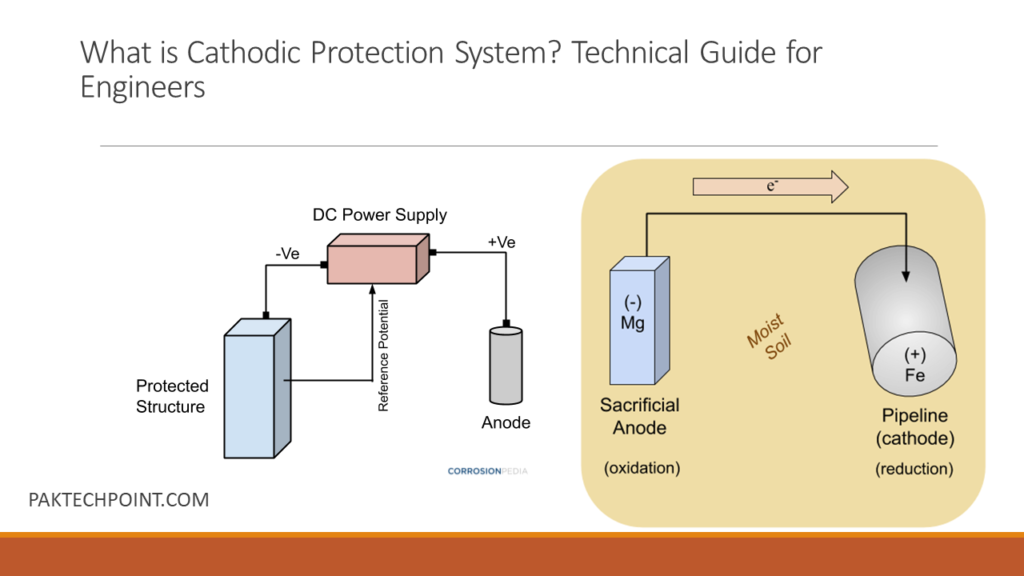
The corrosion of metals occurs due to electrochemical reactions between the metal, moisture, and oxygen in the environment. In a cathodic protection system, a more easily corroded sacrificial metal (anode) is connected to the metal structure to be protected (cathode). This creates a galvanic cell where the sacrificial metal corrodes preferentially, while the protected metal remains relatively unaffected.
There are two main types of cathodic protection systems:
- Galvanic Cathodic Protection (Passive System):
In this method, a sacrificial anode made of a more active metal, such as zinc or aluminum, is connected to the metal structure to be protected. The anode corrodes over time, sacrificing itself to protect the structure. This is commonly used for smaller structures like underground pipelines and storage tanks. - Impressed Current Cathodic Protection (Active System):
In this approach, an external power source, usually a rectifier, is used to apply a direct current between the protected structure and anodes. The anodes are typically made of materials like graphite or mixed metal oxide-coated titanium. The applied current counteracts the corrosive process and prevents the metal from corroding. This method is used for larger structures, such as large pipelines, ships, and offshore platforms.
Cathodic protection systems are widely used in industries such as oil and gas, water and wastewater, marine, and transportation. They help prevent metal deterioration, reduce maintenance costs, and ensure the safety and reliability of critical infrastructure. The design and implementation of a cathodic protection system require a thorough understanding of the environment, materials, and electrochemical principles to ensure effective corrosion prevention.
Do You Know About History of Cathodic Protection?
Cathodic protection, a significant advancement in corrosion control, has an interesting history that dates back to the early 19th century. The concept was first introduced and explored by Sir Humphry Davy, a renowned British chemist, through a series of papers presented to the Royal Society in London in the year 1824. This pioneering work laid the foundation for a technique that would revolutionize the protection of metal structures from corrosion.
In 1824, the initial practical application of cathodic protection was demonstrated on the HMS Samarang, a naval ship. The method involved the use of sacrificial anodes made from iron, which were attached to the copper sheath of the ship’s hull below the waterline. This ingenious approach yielded remarkable results by significantly reducing the rate of corrosion on the copper surfaces. However, an unintended consequence of this protection was the rapid increase in marine growth on the ship’s hull. Normally, when copper corrodes, it releases copper ions that have an anti-fouling effect. The excess marine growth caused interference with the ship’s performance, prompting the Royal Navy to decide against further implementation of cathodic protection.
Cathodic Protection Working Principle
The working principle of cathodic protection is based on the fundamental electrochemical concept of reducing the corrosion rate of a metal surface by manipulating the electrochemical reactions that drive corrosion. Cathodic protection achieves this by making the metal structure to be protected (the cathode) the site of a reduction reaction, which counteracts the natural oxidation (corrosion) reaction.

The primary corrosion process involves the flow of electric current between anodic and cathodic areas on a metal surface immersed in an electrolyte (such as soil or water). In the context of cathodic protection, this process is harnessed and controlled to prevent corrosion. The working principle involves two main methods: galvanic (sacrificial anode) cathodic protection and impressed current cathodic protection.
- Galvanic (Sacrificial Anode) Cathodic Protection:
In this method, a more active or easily corroded metal, known as a sacrificial anode, is electrically connected to the metal structure to be protected. When the sacrificial anode and the metal structure are both immersed in an electrolyte (such as soil or water), the anode undergoes oxidation (corrosion) in preference to the protected metal. As the anode corrodes, it releases electrons into the system, generating a flow of electric current. This current counteracts the natural corrosion process that would have occurred on the protected metal. Common materials used as sacrificial anodes include zinc, aluminum, and magnesium. - Impressed Current Cathodic Protection:
Impressed current cathodic protection is an active method that employs an external power source, often a rectifier, to apply a direct current between the protected metal structure (cathode) and a set of anodes. The anodes are usually made of materials that have a stable electrochemical behavior, such as graphite or mixed metal oxide-coated titanium. The applied current forces the cathodic reaction to occur on the structure, preventing the oxidation reaction (corrosion) from taking place. By controlling the current flow, the corrosion rate of the protected metal can be significantly reduced or eliminated.
In both methods, the goal is to shift the corrosion potential of the metal structure to be protected into a region where the natural corrosion reactions are suppressed. This prevents the metal from deteriorating and extends its service life. The choice between galvanic and impressed current cathodic protection depends on factors such as the size of the structure, the environment, and the level of corrosion protection required.
In summary, cathodic protection works by manipulating the electrochemical reactions occurring on the metal surface to prevent corrosion. It utilizes sacrificial anodes or impressed current systems to ensure that the metal structure remains the site of a reduction reaction, effectively hindering the oxidation (corrosion) process.
Cathode Protection System Components
Cathodic Protection (CP) systems consist of several crucial components that work in tandem to safeguard metal structures from corrosion. These components ensure the effectiveness of the CP process, preserving the integrity and longevity of assets. Let’s delve into the essential components that constitute a cathodic protection system:
- Cathodic Protection Rectifiers:
Rectifiers play a pivotal role in cathodic protection systems by converting alternating current (AC) power from the grid into direct current (DC) output. This DC current is used to drive the cathodic protection process, preventing corrosion by supplying a protective current to the metal structure. The rectifier’s voltage and current output can be adjusted to suit the specific requirements of the protected structure. - Cathodic Protection Reference Electrodes:
Reference electrodes are critical components that enable the monitoring and control of cathodic protection systems. These specialized electrodes establish a reference potential that helps assess the corrosion status of the protected structure. By comparing the potential of the reference electrode to the potential of the protected structure, corrosion engineers can ensure that the structure remains under optimal cathodic protection. - Cable:
High-quality cables are employed to connect the cathodic protection rectifiers, reference electrodes, and other components of the system. These cables ensure the seamless transmission of the DC current and potential measurements, maintaining accurate control and monitoring of the cathodic protection process. - Junction Boxes:
Junction boxes serve as crucial connection points where cables from different components are joined together securely. These boxes provide protection to the connections from environmental factors such as moisture and physical damage. Junction boxes are designed to ensure reliability and longevity of the system’s electrical connections. - Splice Kits:
Splice kits are utilized for creating strong, reliable, and insulated connections between cables in cathodic protection systems. These kits ensure that cable connections are properly insulated, preventing corrosion and ensuring the continuity of the protective current. - Cathodic Protection Test Stations:
Cathodic protection test stations are installed along the protected structure to facilitate routine monitoring and testing of the system’s performance. These stations allow technicians to measure and record potential and current values, which are essential indicators of the cathodic protection system’s effectiveness.
Cathodic Protection Methods
Cathodic protection is a technique used to prevent or control the corrosion of metal surfaces by making the metal structure the cathode of an electrochemical cell. There are two primary methods of cathodic protection: galvanic (sacrificial anode) cathodic protection and impressed current cathodic protection. Each method has its own applications and benefits.
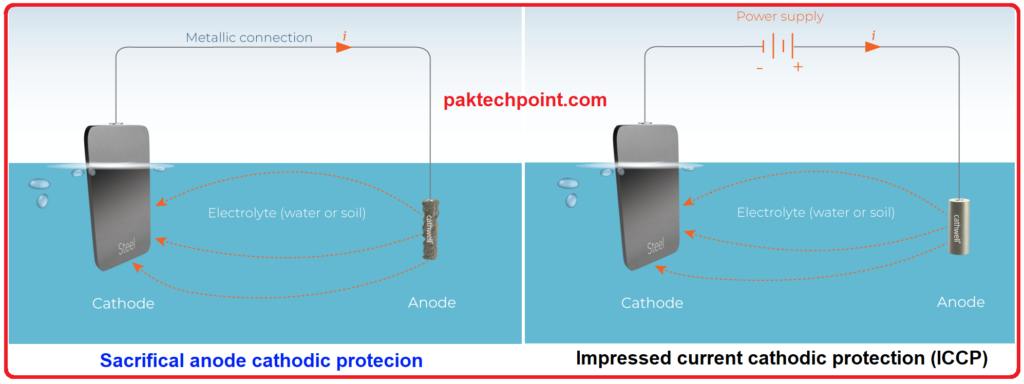
- Galvanic (Sacrificial Anode) Cathodic Protection:
Galvanic cathodic protection, often referred to as sacrificial anode cathodic protection, involves using a more reactive metal (sacrificial anode) that corrodes sacrificially to protect the metal structure to be preserved (cathode). The sacrificial anode is connected to the metal structure, and when they are immersed in an electrolyte (such as soil or water), a galvanic cell is formed. The anode corrodes preferentially to protect the cathode from corrosion.- Advantages:
- Simple and cost-effective method.
- Suitable for small structures or localized protection.
- Minimal maintenance required.
- Disadvantages:
- Limited control over protection level.
- Not suitable for large structures or aggressive environments.
- Advantages:
- Impressed Current Cathodic Protection:
Impressed current cathodic protection involves applying an external direct current (DC) from a power source (rectifier) to the metal structure to be protected. This method is used when sacrificial anodes are not sufficient to provide the required level of protection. The applied current shifts the corrosion potential of the metal structure to a more negative value, preventing corrosion.- Advantages:
- Greater control over protection level.
- Suitable for larger structures and aggressive environments.
- Can be adjusted as needed.
- Disadvantages:
- More complex and expensive to install and maintain.
- Requires a power source and monitoring equipment.
- Advantages:
Both methods have their own applications and are chosen based on factors such as the size of the structure, environmental conditions, and required protection level. The selection of the appropriate method depends on careful evaluation and consideration of these factors to ensure effective corrosion prevention and extended service life for metal structures.

Cathodic Protection Measurements:
Pipe-to-Soil Potential (ON Potential):
Pipe-to-Soil Potential refers to the voltage between a buried pipeline and a reference electrode placed in the soil above it. This measurement is made to understand the corrosive reactions occurring between the pipeline and the surrounding soil (electrolyte). The potential is measured while the cathodic protection system is active, and it provides insights into the protection level of the pipeline against corrosion.
Instant OFF Potential:
During pipe-to-soil potential measurements, the pipeline’s potential may be affected by IR drop errors, leading to inaccurate readings. To counteract this effect, an Instant OFF Potential measurement is used. In this method, the cathodic protection current is briefly interrupted, allowing the true pipe-to-soil potential to be measured without the interference of IR drop. This provides a more accurate assessment of the actual protection applied to the pipeline.
Coupon Current:
Corrosion coupons are used to monitor the effectiveness of a cathodic protection system. A corrosion coupon is a representative sample of the pipeline material buried close to the pipe, exposed to the same environmental conditions. Connected to the pipeline via a test post, it simulates the pipeline’s behavior in the presence of coating defects or “holidays.” By measuring the current flow to/from the coupon, corrosion rates can be determined. Voltage across a shunt is also measured to calculate the current flow.
Role of Instant OFF Potential:
The Instant OFF Potential measurement is crucial to obtaining accurate potential readings. It corrects the potential measurements for IR drop effects that can occur during regular pipe-to-soil potential measurements. By momentarily interrupting the cathodic protection current, a true pipe-to-soil potential is obtained, providing a more precise representation of the actual level of protection on the pipeline.
Measurement Locations:
Cathodic protection measurements, including pipe-to-soil potential, instant OFF potential, and coupon current, can be conducted either at the Transformer Rectifier location or in the field at CP test posts or stations. These measurements offer insights into the cathodic protection system’s performance at specific points along the pipeline and structures, allowing assessment and adjustments to ensure effective corrosion prevention.
These measurement techniques play a crucial role in evaluating the corrosion protection provided by cathodic protection systems. By obtaining accurate data about pipe-to-soil potential, correcting for IR drop effects, and monitoring corrosion coupons, engineers and technicians can ensure the integrity and longevity of pipelines and other metallic structures.
Cathodic Protection Applications
Cathodic protection is a critical technique used to prevent corrosion and extend the service life of various metallic structures and equipment. It finds applications in a wide range of industries and environments where corrosion protection is essential. Here are some common applications of cathodic protection:
- Underground Pipelines and Storage Tanks:
Cathodic protection is extensively used to protect buried pipelines that transport fluids like oil, gas, water, and chemicals. It prevents corrosion of these pipelines by installing sacrificial anodes or impressed current systems. Storage tanks, both above-ground and underground, also benefit from cathodic protection to prevent corrosion on the tank surfaces in contact with the stored substances. - Marine Structures:
Structures such as ship hulls, offshore platforms, piers, docks, and marine piles are exposed to aggressive marine environments. Cathodic protection safeguards these structures from corrosion caused by saltwater and other corrosive agents. Impressed current cathodic protection is commonly employed for larger marine structures. - Ship and Boat Hulls:
Cathodic protection is used on ship hulls to prevent corrosion in seawater. Sacrificial anodes or impressed current systems are attached to the hull’s submerged areas, reducing the need for frequent dry-docking and maintenance. - Steel Reinforced Concrete:
In reinforced concrete structures, the steel reinforcement bars can corrode due to environmental factors or exposure to chloride ions. Cathodic protection is applied to extend the life of these structures, particularly in environments like coastal regions where chloride-induced corrosion is prevalent. - Oil and Gas Facilities:
Cathodic protection is crucial in the oil and gas industry to protect infrastructure such as well casings, production platforms, and refinery equipment. Corrosion prevention is essential to ensure the safety and integrity of equipment handling hazardous substances. - Water Storage and Distribution Systems:
Cathodic protection is used to protect water storage tanks, pipelines, and distribution systems from corrosion due to contact with water, soil, and other corrosive agents. - Power Plants and Industrial Facilities:
Cathodic protection is employed to protect structures within power plants, industrial facilities, and chemical plants that are exposed to harsh chemicals, elevated temperatures, and varying pH levels. - Bridge Structures:
Steel bridges in aggressive environments are susceptible to corrosion. Cathodic protection helps prolong the life of these structures by preventing rust formation and deterioration. - Grounding Systems:
Cathodic protection is used to protect grounding systems of electrical substations and power distribution facilities from corrosion, ensuring effective electrical grounding. - Cathodic Protection Testing and Monitoring:
Cathodic protection systems require regular testing and monitoring to ensure their effectiveness. Techniques such as potential measurements and current density mapping are used to assess the corrosion protection level and identify any maintenance needs.
Cathodic protection plays a critical role in preserving the integrity of a wide range of metallic structures and equipment, contributing to safety, sustainability, and cost savings across various industries.
International Codes and Standards used for Cathodic Protection
Cathodic protection is a specialized field that requires adherence to various international codes and standards to ensure the effective design, installation, operation, and maintenance of cathodic protection systems. These codes and standards provide guidelines and best practices for different aspects of cathodic protection. Here are some of the key international codes and standards commonly used in the field of cathodic protection:
- NACE International:
NACE International (formerly known as the National Association of Corrosion Engineers) is a leading organization in the field of corrosion control and cathodic protection. NACE publishes numerous standards and guidelines related to cathodic protection, including:
- NACE SP0169: “Control of External Corrosion on Underground or Submerged Metallic Piping Systems”
- NACE RP0188: “Discontinuity (Holiday) Testing of New Protective Coatings on Conductive Substrates”
- NACE RP0285: “Corrosion Control of Underground Storage Tank Systems by Cathodic Protection”
- NACE SP0208: “Control of Internal Corrosion in Steel Pipelines and Piping Systems”
- ISO Standards:
The International Organization for Standardization (ISO) also provides relevant standards related to cathodic protection. ISO standards are globally recognized and widely used in various industries. Some ISO standards include:
- ISO 15589-1: “Petroleum, Petrochemical and Natural Gas Industries – Cathodic Protection of Pipeline Transportation Systems – Part 1: On-land Pipelines”
- ISO 15589-2: “Petroleum, Petrochemical and Natural Gas Industries – Cathodic Protection of Pipeline Transportation Systems – Part 2: Offshore Pipelines”
- ASTM International:
ASTM International publishes standards that cover various aspects of cathodic protection materials, testing methods, and practices. Some relevant ASTM standards include:
- ASTM G57: “Test Method for Field Measurement of Soil Resistivity Using the Wenner Four-Electrode Method”
- ASTM G8: “Test Methods for Cathodic Disbonding of Pipeline Coatings”
- ASTM G97: “Test Method for Laboratory Evaluation of Pipeline Coatings and Lining Materials by Autoclave”
- BSI Standards:
The British Standards Institution (BSI) provides standards that are widely used in the United Kingdom and internationally. Some relevant BSI standards include:
- BS EN 12473: “Cathodic Protection of Pipeline Systems”
- BS EN 12954: “Cathodic Protection of Buried or Immersed Metallic Structures – General Principles and Application for Pipelines”
- API Standards:
The American Petroleum Institute (API) publishes standards specific to the oil and gas industry, including those related to cathodic protection:
- API RP 651: “Cathodic Protection of Aboveground Petroleum Storage Tanks”
- API RP 652: “Lining of Aboveground Petroleum Storage Tank Bottoms”
- ICCP Standards:
For impressed current cathodic protection systems, the International Maritime Organization (IMO) has developed guidelines for the design and installation of cathodic protection on ships and marine structures. These guidelines are outlined in the “Guidelines for Control and Monitoring of Marine Cathodic Protection Systems (ICCP Guidelines)” published by the IMO.
It’s important to note that codes and standards may vary based on the specific industry, location, and application. Engineers, designers, and professionals working in cathodic protection should stay updated with the latest versions of these standards and ensure compliance to achieve effective corrosion control and asset protection.
Read Also:
- Cathodic Protection Design Package Preparation SABP-X-001 Download
- CATHODIC PROTECTION INSTALLATION DETAILS
- CATHODIC PROTECTION INSTALLATION METHOD STATEMENT
- Cathodic Protection Commissioning Reports
- Specific Terms Related to Cathodic Protection