The failure modes of VRLA (Valve-Regulated Lead-Acid) batteries have several key challenges. Dry failure mode involves water loss and dehydration, often due to low gas recombination efficiency, moisture infiltration, grid corrosion, and self-discharge. Premature capacity loss results from improper cycling, lack of additives, low-rate material use, and assembly issues.
Thermal runaway can occur with excessive heat generated inside the battery, attributed to oxygen recombination, limited gas venting, high temperatures, uncontrolled charging, and poor cooling.
Grid corrosion, especially the positive grid, can lead to elongation, deformation, and short circuits, often necessitating thicker grids to compensate. Preventive measures include selecting appropriate float voltages, proper cycling practices, monitoring additives, maintaining material density, and employing temperature compensation and ventilation to enhance VRLA battery performance and longevity.
Failure Modes of VRLA Battery
1. Dry Failure Mode
Dry failure mode in VRLA (Valve-Regulated Lead-Acid) batteries and the various factors that contribute to battery dehydration and failure.
1. Gas Recombination Efficiency:
- Gas recombination efficiency refers to the ability of the battery to recombine the hydrogen and oxygen gases that are generated during its operation. These gases are recombined to form water, which helps maintain the battery’s electrolyte level.
- If the selected float voltage is too low, it may result in less oxygen precipitation and higher gas recombination efficiency. However, this can lead to negative electrode salinization and a shortened battery life due to prolonged undercharging.
- Conversely, if the float voltage is set too high, gas precipitation increases, and gas recombination efficiency decreases. While this may prevent negative electrode failure, it can cause the safety valve to open frequently, increasing water loss, and may also lead to positive grid corrosion, further affecting battery life.
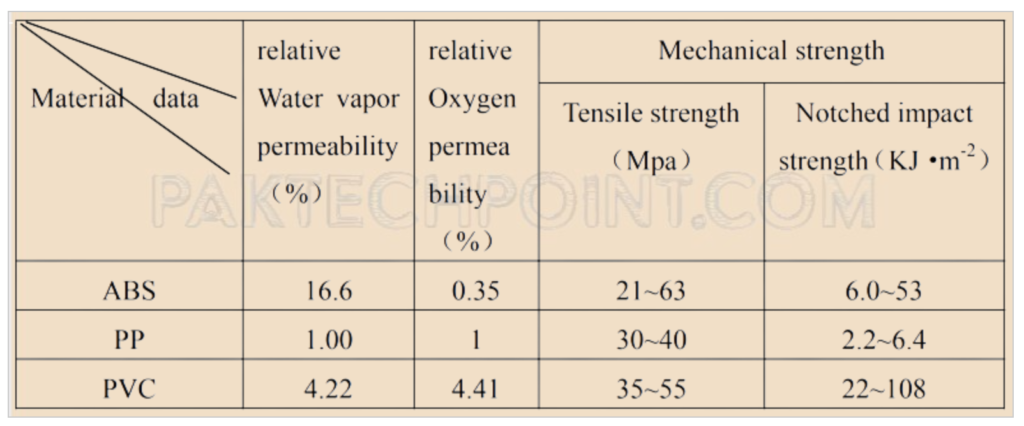
2. Moisture Penetration from the Housing Material:
- The text mentions that the choice of battery shell material can affect moisture penetration. ABS materials, while having good strength, have relatively high water vapor permeability.
- The penetration of moisture into the battery case depends on factors such as the type and thickness of the housing material, the nature of the material, and the vapor pressure difference between the inside and outside of the housing.
3. Grid Corrosion:
- Grid corrosion is a process where the lead grids in the battery corrode, and this corrosion consumes water. The reaction involved is:
Pb + 2H2O -> PbO2 + 4H+ + 4e_ - This corrosion process can lead to a loss of water in the battery and contribute to dehydration.
4. Self-Discharge:
- Self-discharge is a phenomenon where a battery loses charge over time even when not in use. In the case of VRLA batteries, during self-discharge, oxygen is exhausted from the anode (positive electrode) and combines with the negative electrode.
- However, hydrogen exhausted from the negative electrode cannot combine at the positive electrode. Instead, it accumulates in the battery, which can lead to gas release from the safety valve and the loss of water.
- Self-discharge tends to occur more rapidly at higher temperatures, which can exacerbate water loss from the battery.
2. Failure Mode of Premature Capacity Loss
The failure mode of premature capacity loss in VRLA (Valve-Regulated Lead-Acid) batteries, particularly those that use low-antimony or antimony-free grid alloy. Premature capacity loss means that the battery’s ability to hold and deliver its rated capacity diminishes earlier than expected. Here are the conditions and factors that contribute to this type of failure:
- Inappropriate Cycling Conditions:
- Continuous high-rate discharge: If the battery is subjected to continuous high-rate discharges, it can lead to premature capacity loss. High-rate discharges put significant stress on the battery and can degrade its capacity over time.
- Deep discharge: Deep discharges, where the battery is discharged to very low levels, can also accelerate capacity loss. Repeated deep discharges can be particularly harmful to battery health.
- Charging begins with low current density: Charging a battery with a low current density (slow charge) can contribute to capacity loss, especially if the battery is frequently charged in this manner.
- Lack of Special Additives:
- The absence of special additives such as antimony (Sb), tin (Sn), or phosphoric acid (H3PO4) in the battery’s composition can lead to premature capacity loss. These additives can improve the performance and longevity of lead-acid batteries.
- Low-Rate Utilization of Active Material:
- When the active material in the battery is underutilized, it can result in premature capacity loss. Factors contributing to low-rate utilization include excess electrolyte and plates that are too thin. Inefficient use of active material reduces the battery’s overall capacity.
- Low Density of Active Material:
- Low-density active material means that there is less lead material available for the chemical reactions that occur within the battery. This can lead to reduced capacity. Low-density active material can result from factors like low-quality materials or inadequate assembly processes.
- Assembly Pressure:
- If the battery’s plates are assembled with insufficient pressure, it can result in poor contact between the active material and the grid. This poor contact can lead to capacity loss and decreased battery performance.
Premature capacity loss is a significant concern for VRLA batteries, especially when certain conditions or factors are not properly managed. Proper cycling practices, the use of appropriate additives, maintaining the right density of active material, and ensuring adequate assembly pressure are all critical factors in preventing this type of failure and extending the lifespan of VRLA batteries.
3. Failure Mode of Thermal Runaway
Here discusses the failure mode of thermal runaway in VRLA (Valve-Regulated Lead-Acid) batteries. Thermal runaway is a critical issue in battery systems, and it can lead to catastrophic failure and safety hazards. Here are the key points from the text regarding the causes and prevention of thermal runaway:
Causes of Thermal Runaway:
- Recombination Process: The oxygen recombination process within VRLA batteries generates heat. This process allows for more heat to be generated within the battery as oxygen is recombined.
- Limited Exhaust of Gas: VRLA batteries have limited exhaust of gas, which reduces the dissipation of heat. This limited gas exhaust can contribute to heat buildup within the battery.
- High Ambient Temperatures: When the working environment of VRLA batteries is too hot, or if the charging device malfunctions and charges the battery too rapidly, the internal temperature of the battery can increase.
- Poor Cooling: Inadequate cooling of the battery can exacerbate the overheating issue. As the internal temperature increases, the battery’s cooling capability may become less effective.
- Decreased Internal Resistance: As the battery temperature rises, its internal resistance decreases. This, in turn, can lead to an increase in charging current, creating a vicious cycle of further temperature rise.
Preventing Thermal Runaway:
To prevent the occurrence of thermal runaway and manage the heat-related risks associated with VRLA batteries, the following measures are recommended:
- Temperature Compensation: Charging equipment should have a temperature compensation function or limit. This means that the charging process should be adjusted based on the ambient temperature to prevent rapid charging in high-temperature conditions.
- Quality Control Valve: Ensure that the quality control valve of the battery functions correctly, allowing for the normal discharge of gas from inside the battery. Proper gas venting helps manage internal pressure and heat.
- Ventilation: Place VRLA batteries in well-ventilated positions to allow heat to dissipate effectively. Adequate ventilation can help maintain a safer operating temperature for the batteries.
- Temperature Control: Monitor and control the battery temperature within safe limits. This can include using temperature sensors and implementing cooling systems when necessary.
- Prevent Rapid Charging: Avoid rapid and uncontrolled charging of the battery. Control the rate of charge to prevent overheating and thermal runaway.
Thermal runaway is a serious safety concern, especially in battery systems used in critical applications. Proper design, monitoring, and maintenance are essential to minimize the risk of thermal runaway and ensure the safe operation of VRLA batteries.
4. Negative irreversible sulfation:
Here is the concept of negative irreversible sulfation in lead-acid batteries, particularly in the context of improper usage and maintenance. Sulfation is a common issue in lead-acid batteries, and it refers to the formation of lead sulfate crystals on the battery plates during discharge. These crystals can typically be reduced back to lead during the charging process, which is a reversible process.
However, if a lead-acid battery is not properly used and maintained, especially in cases of insufficient charging or over-discharge, the lead sulfate on the negative electrode can become thicker and harder. This thick and hard lead sulfate is challenging to convert back to its active form through conventional charging methods. As a result, it accumulates and persists on the electrode, leading to a reduction in battery capacity and, in severe cases, potentially causing the battery to reach the end of its usable life. This condition is referred to as “plate irreversible sulfation.”
To prevent negative irreversible sulfation in lead-acid batteries, it is essential to follow proper usage and maintenance practices, which include:
- Timely Charging: Ensure that the battery is charged promptly after use and not left in a discharged state for extended periods. Charging helps prevent the formation and buildup of thick lead sulfate crystals.
- Avoid Over-Discharge: Avoid over-discharging the battery, as excessive discharge can accelerate the formation of lead sulfate. Implement measures to prevent the battery from reaching critically low voltage levels.
- Proper Charging: Use appropriate charging methods and ensure that the battery is charged to the manufacturer’s recommended voltage levels. Proper charging helps to dissolve lead sulfate and maintain battery capacity.
5. Grid Corrosion and Elongation
This section we are going to discuss grid corrosion and elongation in lead-acid batteries, particularly focusing on the positive grid and its impact on battery performance. Here are the key points:
- Positive Grid Thickness: The positive grid in lead-acid batteries is typically made thicker than the negative grid. One of the reasons for this is that during charging, especially during overcharging, the positive plate grid can corrode. As a result, the lead dioxide on the positive plate grid gradually loses its function, and the grid may need to compensate for the amount of corrosion by being thicker.
- Impact of Float Voltage: It’s crucial to select the right float voltage for the battery based on the ambient temperature. If the float voltage is set too high, it can accelerate the loss of water in the battery and also lead to an accelerated corrosion of the positive grid.
- Grid Corrosion Consequences: When the positive grid alloy corrodes, it creates stress within the battery. This stress can lead to the deformation and elongation of the battery plates. If this elongation is severe, it can result in short circuits either at the edge of the plates or between the top plate and the bus.
- Battery Life Dependence: The life of a VRLA battery is closely tied to the life of the positive plate. Battery design life is calculated based on the rate of corrosion of the positive grid alloy. The more the positive grid corrodes, the less remaining capacity the battery has, leading to a shorter battery life.
In summary, positive grid corrosion in lead-acid batteries, particularly due to overcharging or high float voltage, can lead to physical changes in the battery plates, such as elongation and deformation. These changes can ultimately result in short circuits and reduce the battery’s capacity and lifespan. Proper maintenance, including monitoring and controlling the charging process, is essential to mitigate these issues and maximize the life of lead-acid batteries.