What is Galvanic Corrosion?
Galvanic corrosion is indeed a common and widely studied type of corrosion. It occurs when two dissimilar metals or materials come into contact in the presence of an electrolyte (such as water or a corrosive medium). This contact creates a galvanic cell, which leads to the flow of electric current between the two materials, resulting in the corrosion of one of them. Here are some key points about galvanic corrosion:
- Common Occurrences: Galvanic corrosion can be found in various scenarios, as mentioned:
- Plumbing: Copper pipes connected to steel pipes.
- Electronics: Different metals and semiconductors in microelectronic devices.
- Composite Materials: Metal matrix composites with reinforcing materials.
- Marine Environments: Ships with various metal components in contact with water.
- Rate of Corrosion: The rate of galvanic corrosion can vary widely based on factors such as the types of metals involved, the nature of the electrolyte, and the surface area of contact. In some cases, it can lead to rapid deterioration.
- Complex Nature: While galvanic corrosion is qualitatively understood, it is highly complex due to the interplay of electrochemical reactions between different metals. Quantifying its effects has been challenging.
- Cathodic Protection: Interestingly, galvanic corrosion can sometimes provide corrosion protection to one of the metals involved. This is the principle behind cathodic protection using sacrificial anodes. In this method, a more reactive metal is intentionally used, and it corrodes sacrificially to protect the less reactive metal.
- Advances in Understanding: With the advancement of computer technology and the development of specialized software, there have been significant strides in understanding and predicting galvanic corrosion. Computational methods allow for more precise modeling of the electrochemical processes involved.
- Corrosion Science: Galvanic corrosion is an essential aspect of corrosion science, which involves the study of the electrochemical, chemical, and physical processes that lead to the deterioration of materials. Understanding these processes is crucial for preventing and mitigating corrosion in various industries.
Galvanic corrosion highlights the importance of material selection and design considerations in industries where dissimilar metals or materials come into contact. Proper preventive measures, such as coatings, insulating materials, or cathodic protection systems, can be employed to minimize the impact of galvanic corrosion and extend the lifespan of materials and structures.
Galvanic Corrosion Occurrence:
Galvanic corrosion occurs when dissimilar metals are electrically coupled and exposed to an electrolyte. It results in corrosion of the anodic member, and the difference in corrosion rates between the coupled and uncoupled states is called the difference effect. In cases where the galvanic current protects the cathodic member, it is referred to as cathodic protection. Understanding these principles is crucial for managing corrosion in engineering and material applications.
- Galvanic Current: When two dissimilar conducting materials come into electrical contact and are exposed to an electrolyte (typically a corrosive medium), a current, known as the galvanic current, flows between them. This electric current is a fundamental aspect of galvanic corrosion.
- Galvanic Corrosion: Galvanic corrosion specifically refers to the corrosion that occurs at the anodic member (the more reactive metal) of such a coupled system. This type of corrosion is directly associated with the galvanic current and follows Faraday’s law, which describes the relationship between the amount of material corroded and the electric current.
- Local Corrosion: Under conditions where two dissimilar materials are electrically coupled, any additional corrosion occurring on the anodic member (the one more prone to corrosion) is termed “local corrosion.” This corrosion can either match or differ from the corrosion that would occur if the two metals were not electrically connected.
- Normal Corrosion: Normal corrosion is the corrosion that takes place on a material when it is not electrically connected to another dissimilar material. It serves as a baseline to compare with local corrosion when the two materials are coupled.
- Difference Effect: The “difference effect” is the variation or contrast between the local corrosion (corrosion on the anodic member of the couple) and normal corrosion (corrosion without electrical coupling). This difference can be either positive (greater corrosion in the coupled system) or negative (less corrosion in the coupled system).
- Cathodic Protection: In situations where a galvanic current flows, it often leads to a reduction in the total corrosion rate of the cathodic member (the less reactive metal) of the couple. This effect is known as cathodic protection. Essentially, the cathodic member is protected from corrosion by the presence of the more reactive anodic member.
C. FACTORS IN GALVANIC CORROSION
Several factors come into play, and they can be categorized into different groups. These factors contribute to the complexity of galvanic corrosion:
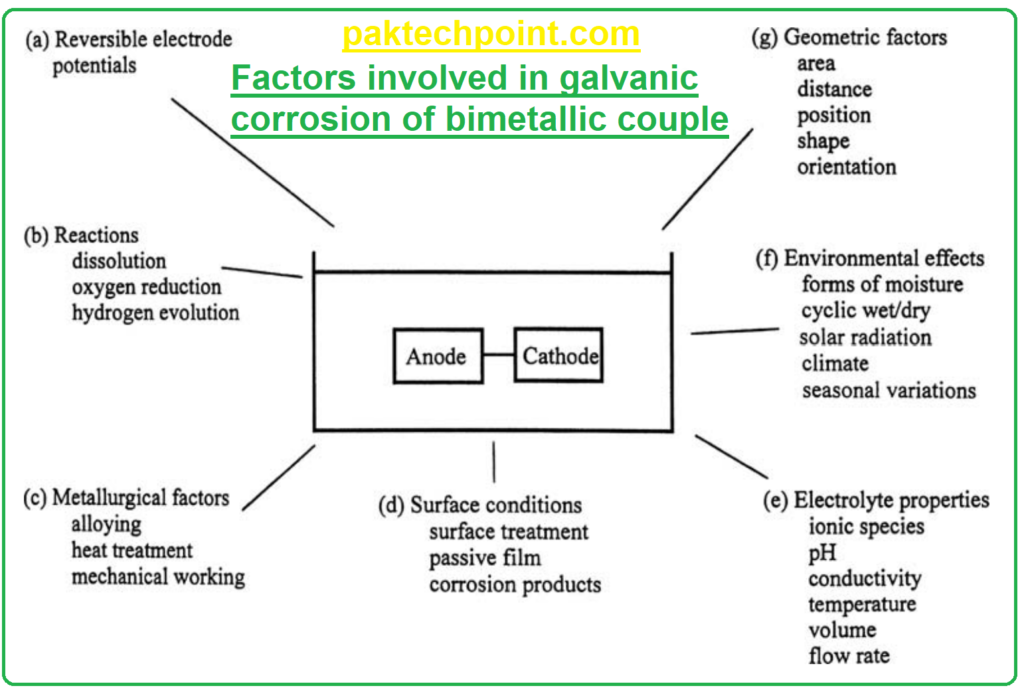
Factors Affecting Galvanic Corrosion:
(a) Potential Difference: The potential difference (voltage) between the two coupled metals is a fundamental factor driving galvanic corrosion. Metals with a higher potential difference exhibit more significant galvanic effects.
(b) Material Properties: The inherent properties of the metals involved, such as their electrochemical behavior, corrosion resistance, and composition, play a crucial role in galvanic corrosion.
(c) Environmental Conditions: The environment in which the materials are placed has a significant impact. Factors like temperature, humidity, acidity or alkalinity of the electrolyte (corrosive medium), and the presence of specific ions can influence galvanic corrosion.
(d) Geometric Factors: The physical arrangement and geometry of the coupled metals can affect the galvanic corrosion rate. These factors include the surface area of each metal in contact, the distance between them, and the shape of the materials.
(e) Surface Conditions: The condition of the metal surfaces, including their cleanliness, roughness, and the presence of protective coatings or oxides, can influence galvanic corrosion.
(f) Electrode Surface Kinetics: The kinetics of the electrochemical reactions occurring at the electrode surfaces can be complex and challenging to determine. Reaction rates and how they change over time are factors to consider.
(g) Flow and Agitation: The movement or flow of the electrolyte can affect galvanic corrosion. For instance, increased flow or agitation of the electrolyte can lead to a more uniform corrosion rate.
(h) Temperature: Temperature variations can influence galvanic corrosion by affecting reaction rates and the solubility of corrosion products.
(i) Differential Aeration: Differences in oxygen concentration in the electrolyte near the two coupled metals can create variations in corrosion rates.
(j) Galvanic Series: The position of metals in the galvanic series (a list of metals ranked by their tendency to corrode) is essential in predicting which metals are more likely to corrode when coupled.
(k) Polarization Effects: The applied electrical potential or polarization can also influence galvanic corrosion. In some cases, polarization can mitigate or enhance corrosion.
It’s important to note that while some factors in categories (a) to (c) are relatively consistent for a given couple in various situations, factors in categories (d) to (g) can vary widely and can be more challenging to predict and control. Galvanic corrosion is indeed more complex than normal corrosion due to its interaction with geometric and environmental factors, making it a subject of detailed study and analysis in materials science and corrosion engineering.
D. MATERIAL FACTORS
D1. Effects of Coupled Materials
Galvanic corrosion involves the interaction of different materials in the presence of an electrolyte. Various factors affect this corrosion phenomenon, including the properties of the materials involved, potential differences, and reaction kinetics. Here are the key considerations:
- The intrinsic polarity of a galvanic couple is determined by the reversible electrode potentials of the coupled metals. However, the actual corrosion potential of a metal in an electrolyte can differ significantly from its thermodynamic equilibrium value due to kinetic processes.
- Galvanic corrosion rates are not solely dependent on the potential difference between metals. Metals with smaller potential differences can sometimes exhibit higher corrosion rates than those with larger differences.
- Reaction kinetics at electrode surfaces, such as the rate of oxygen reduction and diffusion, play a crucial role in galvanic corrosion.
- The condition of metal surfaces, including cleanliness, roughness, and the presence of protective coatings or oxide layers, can influence galvanic corrosion rates.
- Complex microstructures in alloys with multiple phases can lead to microscale galvanic action, resulting in localized corrosion.
- The addition of alloying elements to a metal may not significantly affect its reversible potential but can alter the kinetics of electrochemical processes, impacting behavior in galvanic action.
- In some cases, galvanic coupling can increase the corrosion rate of the cathodic member due to changes in alkalinity near its surface.
- Galvanic corrosion is not limited to bimetallic couples and can occur when metals interact with nonmetallic materials, such as composites or minerals.
- Minerals often exhibit noble potentials compared to metals, potentially causing galvanic corrosion of metals used in processing equipment when in contact with conducting minerals.
In summary, galvanic corrosion is a complex phenomenon influenced by material properties, electrochemical behavior, and environmental factors. While potential differences between materials are important, other factors like reaction kinetics and surface conditions also significantly impact the corrosion rate in galvanic couples. Researchers continually study this complex corrosion process.
D2. Effect of Area
The effect of the surface area of anode and cathode materials plays a crucial role in galvanic corrosion, and its significance depends on the type of control within the system. Let’s delve into this aspect in more detail:
![FIGURE 10.2. Effect of area of mild steel cathode on weight loss of
Zn anode (area of 100 cm2
) and on number of coulombs flowing
between Zn–steel couple over a 96-h period in 1N NaCl solution at
25C [38].](https://paktechpoint.com/wp-content/uploads/2023/08/image-196-1024x873.png)
In galvanic systems, the corrosion rate can be influenced by the relative surface areas of the anode (the corroding metal) and the cathode (the metal that is protected or corroded less). The impact of surface area variation depends on whether the galvanic system is under cathodic or anodic control.
- Cathodic Control: When the galvanic system is under cathodic control, changes in the surface area of the anode have little effect on the overall corrosion rate. However, variations in the cathode’s surface area can significantly influence the corrosion rate.
- For example, if you have a system where iron is the anode (corroding) and zinc is the cathode (protected or corroded less), increasing the surface area of the zinc cathode will have a substantial effect on the corrosion rate. This means that the galvanic corrosion of zinc is primarily influenced by the cathode’s surface area.
- Anodic Control: Conversely, when the system is under anodic control, adjustments to the cathode’s surface area have minimal impact on the total corrosion rate. Instead, variations in the anode’s surface area become more significant.
- Consider a scenario where zinc is the anode and iron is the cathode. In this case, changes in the zinc anode’s surface area will only slightly affect the corrosion rate. This indicates that the galvanic corrosion of zinc in this system is predominantly influenced by the anode’s surface area.
In many situations, galvanic currents are directly proportional to the surface area of the cathode. For instance, if you increase the cathode area, the galvanic corrosion rate of the anode material tends to increase as well. This phenomenon is observed in various galvanic systems.
To illustrate, let’s revisit the example of zinc corrosion. The galvanic corrosion of zinc is shown to increase as the surface area of the iron cathode (cathodic material) increases. This suggests that the galvanic corrosion of zinc in this specific system is primarily under cathodic control.
Similar observations have been made for aluminum alloys when coupled with materials like copper, stainless steels, or Ti–6Al–4V. In these cases, the total galvanic current remains independent of the anode’s surface area but is directly proportional to the cathode’s surface area.
D3. Effect of Surface Condition
The surface condition of metals when exposed to an electrolyte, such as in galvanic corrosion scenarios, is a critical factor that significantly influences the electrochemical processes and corrosion behavior. Here are key points regarding the effect of surface condition on galvanic corrosion:
- Surface Films: Metals in contact with an electrolyte are seldom in a bare state; they are typically covered by surface layers. These layers can include adsorption layers or solid surface films. This is a crucial distinction as it creates differences between the intrinsic polarity (theoretical) and apparent polarity (actual) of the metal surfaces. The formation of these surface films, whether they are composed of salts or oxides, can substantially alter the electrochemical properties of the metal surfaces, leading to variations in galvanic corrosion behavior.
- Corrosion Product Films: Surface films can act as physical barriers between the metal surface and the surrounding environment. Moreover, if these films can conduct electrical current, either as conductors or semiconductors, they may actively participate in electrochemical reactions. Many common corrosion products, like metal oxides, are conductive materials, primarily functioning as semiconductors. The electronic structure of these oxide films often results in potentials that differ significantly from those of the base metals themselves.
- Impact on Galvanic Corrosion: The presence of such oxides or surface films can have a profound impact on galvanic corrosion rates. In fact, in many cases, it is these oxides, rather than the base metals, that predominantly determine the electrode potential and the metal’s position in a galvanic series. Certain oxides, characterized by high cathodic efficiency and noble potential values within a galvanic couple, can lead to a more pronounced galvanic corrosion rate of the coupled metal.
- Surface Passivation: Surface passivation, the development of a protective film that prevents further corrosion, is significant in galvanic corrosion processes. For example, aluminum is often passivated in neutral aqueous solutions. However, the extent of passivity can be limited in solutions containing specific ions, such as chloride ions, and may break down under certain conditions. This can result in complex galvanic behavior, where aluminum can act as an anode or cathode depending on the solution it is exposed to.
- Partial Surface Coverage: When a surface film doesn’t fully cover the entire metal surface, a local galvanic cell can form. In such cases, the passivated part of the metal surface acts as the cathode, while the non-passivated area experiences higher corrosion rates. This scenario can potentially lead to pitting corrosion, where localized, intense corrosion occurs.
- Time Effects: Time is an essential factor in considering galvanic corrosion. Over time, changes occur both in the physical structure and chemical composition of the corroding metal surface and in the composition of the surrounding solution. These changes can include alterations in surface roughness, adsorption of species, formation of passive films, precipitation of solid layers, and depletion of reactants. These changes may lead to variations in equilibrium potentials, the types of reactions occurring, rate-controlling processes, and more. Consequently, corrosion potential can vary significantly based on the nature and extent of these changes.
- Temporal Variation: Galvanic corrosion rates may change with time due to fluctuations in polarity and potential differences between the metals in the couple. Studies have shown that potentials of various bimetallic couples, when exposed to environments like seawater, can be highly variable, and galvanic currents may decrease significantly within the initial days of exposure.
![FIGURE 10.3. Corrosion potentials and oxygen reduction rates of metal oxides. PFE: passive film
electrode; TOE: thermal oxide electrode [57], (Copyright ASTM. Reprinted with permission.)](https://paktechpoint.com/wp-content/uploads/2023/08/image-197-1017x1024.png)
In summary, the surface condition of metals in galvanic corrosion systems, including the presence of surface films and their conductive properties, has a profound influence on the electrochemical processes and corrosion rates. These factors, in conjunction with time-dependent changes, contribute to the complexity of galvanic corrosion behavior.
Environmental factors
Environmental factors play a crucial role in galvanic corrosion, and the specific conditions of the environment can significantly influence the electrochemical properties and corrosion behavior of metal couples. Here are key points regarding the effects of the environment on galvanic corrosion:
E1. Effects of Solution on Galvanic Corrosion
The environment in which two dissimilar metals interact plays a pivotal role in galvanic corrosion. This electrochemical phenomenon is highly sensitive to the surrounding solution. Let’s delve into the effects of the solution on galvanic corrosion and how various factors come into play.
1. Variability in Electrolytes:
- Metal Potential Variation: Different electrolytes can influence the electrode potentials of metals. Each metal exhibits a specific potential in a given electrolyte. This means that a metal may have varying tendencies to corrode or remain protected depending on the solution it is immersed in.
- Galvanic Series Specific to Environments: A galvanic series, which indicates the relative positions of metals in terms of their corrosion tendencies, is environment-specific. The same metals may have different positions in the galvanic series when exposed to different solutions.
2. Solution Composition:
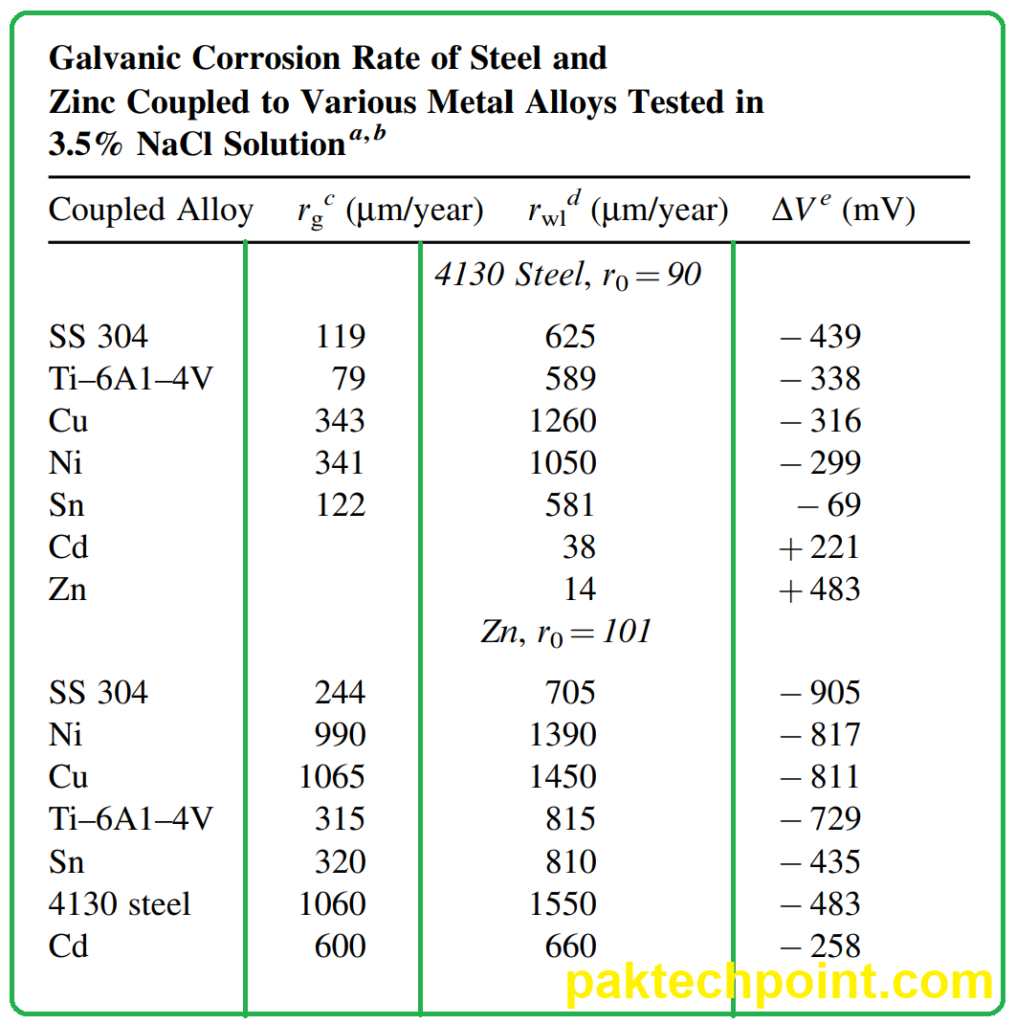
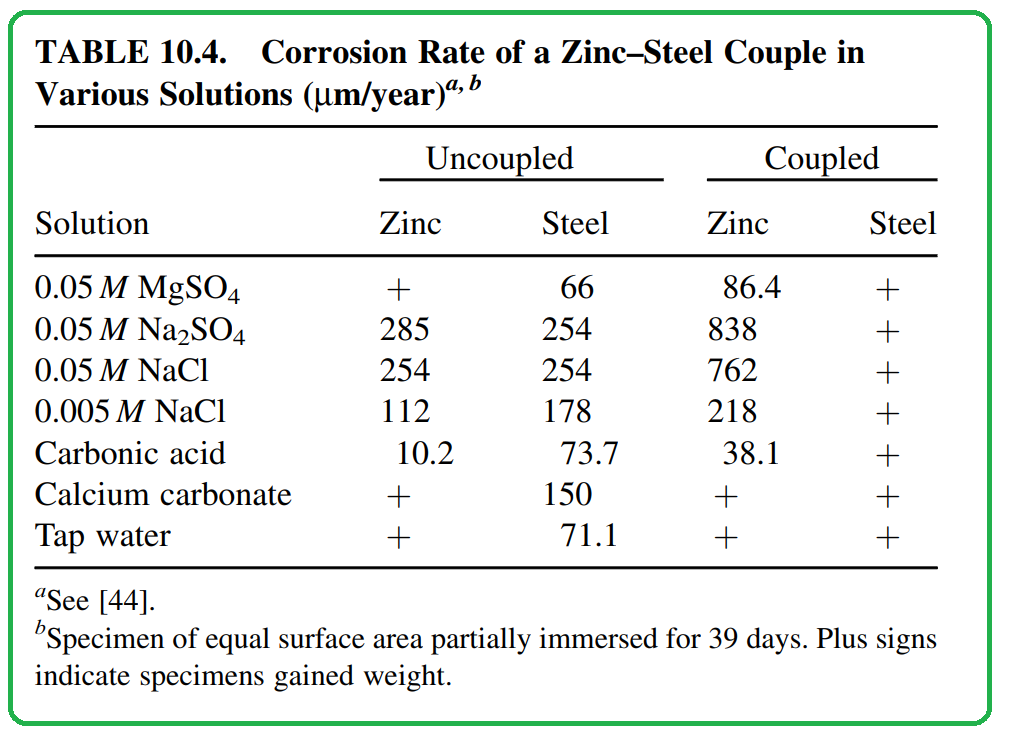
- Effect on Corrosion Rates: The composition of the solution can significantly impact the rate of galvanic corrosion. In Table 10.4, the corrosion rates of zinc and steel in various solutions demonstrate this. Galvanic action typically results in the protection of the more noble metal (steel in this case), but the extent of zinc corrosion varies depending on the solution’s composition.
- Influence of Cations: Cations present in the solution can influence the kinetics of the corrosion reaction. The difference in corrosion rates observed in magnesium sulfate and sodium sulfate solutions highlights the notable effect of cations on galvanic corrosion.
3. Conductivity Matters:
- Uniform vs. Concentrated Corrosion: The conductivity of the electrolyte plays a crucial role in determining how galvanic corrosion is distributed across the surface of the anodic metal. In highly conductive environments, such as seawater, the corrosion is uniformly spread across the metal’s surface. However, as conductivity decreases, galvanic corrosion tends to concentrate in a narrow region near the junction of the two metals. This phenomenon is illustrated in Following Figure.
- Poor Conductivity Reduces Corrosion: Surprisingly, in some cases, galvanic corrosion can be less severe in poorly conducting electrolytes compared to highly conductive ones.
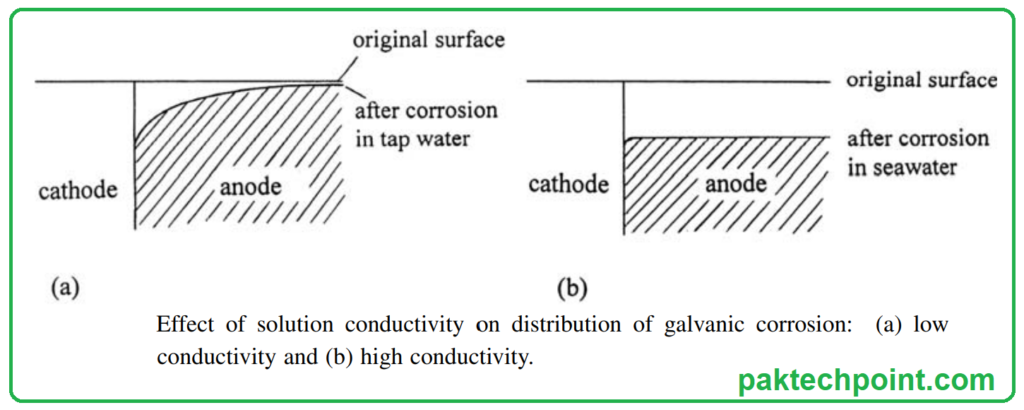
4. Role of Noble Elements:
- Precipitation-Induced Galvanic Cells: Ions of noble elements present in the solution can induce galvanic corrosion of a less noble metal. This occurs when the noble element precipitates and forms small galvanic cells, initiating corrosion. These unexpected interactions can complicate galvanic corrosion predictions.
The nature of the solution in which two dissimilar metals are immersed significantly influences galvanic corrosion. Variability in electrode potentials, solution composition, conductivity, and the presence of noble elements all contribute to the intricate behavior of galvanic corrosion. Understanding these effects is essential for predicting and managing galvanic corrosion in diverse environments.
In summary, environmental factors, including the specific solution characteristics, composition, conductivity, presence of noble ions, changes in electrolyte composition over time, electrolyte thickness, and physical placement of metal couples, all play a vital role in determining the extent and nature of galvanic corrosion. These factors make galvanic corrosion behavior highly complex and environment-dependent.
The behavior of galvanic corrosion is not solely determined by the nature of the electrolyte; the physical characteristics and thickness of the electrolyte layer also play significant roles. Here, we explore how the availability and thickness of the electrolyte can impact galvanic corrosion.
1. Limited Electrolyte Volume:
- Change in Composition: In situations where there’s only a limited amount of electrolyte, the composition of that electrolyte can undergo substantial changes due to the electrochemical reactions taking place. For instance, Massinon et al. noted an increase in pH in a confined electrolyte after a period of galvanic action involving a zinc–steel couple. This change in pH can have far-reaching consequences on the corrosion processes.
2. Thin-Layer Electrolytes in Atmospheric Environments:
- Multiple Effects: Thin-layer electrolytes, often encountered in atmospheric environments, introduce several important factors influencing galvanic corrosion.
- Lateral Resistance: The thickness of the electrolyte affects the lateral resistance it presents. This resistance, in turn, impacts how the electric potential and galvanic current are distributed across the surface of the coupled metals.
- Oxygen Transport: The rate at which oxygen can move through the electrolyte layer is influenced by its thickness. This, in turn, affects the rate of the cathodic reaction, a crucial factor in galvanic corrosion.
- Volume and Solvation Capacity: Thin-layer electrolytes also alter the volume and solvation capacity of the electrolyte. This has implications for the formation of corrosion products, which can further affect the corrosion process.
3. Effect on Potential and Current Distributions:
- Greater Changes in Thinner Electrolytes: Thinner electrolytes result in more significant changes in potential and galvanic current across the surface of a metal. This is primarily due to the increased electrical resistance associated with thinner layers.
- Anode-Cathode Boundary: Galvanic corrosion is most intense near the anode area, particularly at the anode–cathode boundary, when a thin-layer electrolyte is present. This region experiences the highest corrosion rates, while corrosion away from this boundary is minimal, as illustrated in Figure 10.4(a).
- Rate-Controlling Processes: The thickness of the electrolyte layer also determines the rate-controlling process in a given cell dimension. For thinner electrolytes, the galvanic current is more substantial when the anode and cathode are close together. Conversely, in situations where the two electrodes are far apart, the rate-limiting process shifts from oxygen diffusion at close distances to ohmic conduction in the electrolyte at larger distances, as shown in Figure 10.5.
![FIGURE 10.5. Galvanic current as function of distance between
zinc and steel in 0.001 M Na2SO4 solutions of different electrolyte
thickness [80]. [Reprinted from Corrosion Science, 34, X. G. Zhang
and E. M. Valeriote, “Galvanic Protection of Steel and Galvanic
Corrosion of Zinc under Thin Layer Electrolytes,” p. 1957 (1993),
with permission from Elsevier Science.]](https://paktechpoint.com/wp-content/uploads/2023/08/image-201-1024x943.png)
4. Physical Position in Solution:
- Impact on Galvanic Action: The physical position of a galvanic couple within a solution can significantly affect the galvanic action between the coupled metals. For instance, Shams El Din et al. observed a substantial potential variation near the solution surface between a zinc anode and a copper cathode that were partially immersed in the solution. This variation was attributed to the higher concentration of oxygen near the surface compared to the bulk solution.
In summary, the thickness and availability of electrolyte are critical factors influencing galvanic corrosion. Limited electrolyte volume can lead to compositional changes, while thin-layer electrolytes in atmospheric environments affect lateral resistance, oxygen transport, and the formation of corrosion products. Understanding these effects is vital for predicting and managing galvanic corrosion in various scenarios.
E2. Atmospheric Environments
Galvanic corrosion is a common occurrence in atmospheric environments, especially in structures exposed to indoor and outdoor conditions where different materials come into contact. Testing and study of galvanic corrosion in atmospheric settings began as early as 1931 by organizations like the American Society for Testing and Materials (ASTM). Numerous exposure programs worldwide have contributed to understanding this phenomenon, and a comprehensive review by Kucera and Mattsson has discussed various aspects of atmospheric galvanic corrosion.
Measurement of weight loss is often employed to evaluate galvanic corrosion in atmospheric environments. While potentials and galvanic currents can be measured for coupled metals in other settings, it’s challenging to measure in-situ potentials under atmospheric conditions due to the thin layer of electrolyte.
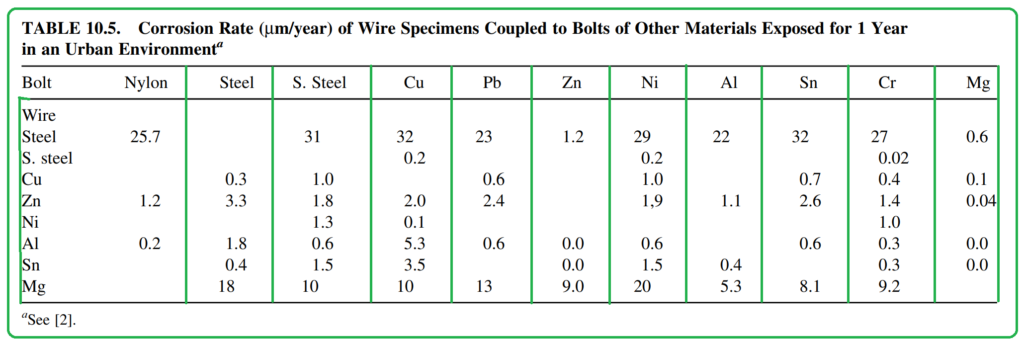
Table 10.5, compiled by Kucera and Mattsson, provides data on galvanic corrosion rates for different metal alloys. These rates are observed when wires are coupled to bolts made of various metals. Interestingly, the galvanic corrosion rate of the wire can be significantly higher than the normal corrosion rate depending on the type of bolt metal and the atmospheric conditions. For instance, zinc’s galvanic corrosion rate can be up to five times greater than normal in a rural atmosphere and three times higher in a marine atmosphere. Notably, the observed corrosion rates don’t always correlate with the differences in reversible potentials of the coupled metals. In fact, steel and copper, acting as cathodic members, tend to cause more galvanic corrosion in the coupled anodic material.
In atmospheric environments, galvanic corrosion tends to be confined to a narrow region near the junction of the anode metal. This limited spread occurs due to the high resistance of thin-layer electrolytes formed by rain and water condensation. Even for metals with significant incompatibility, direct galvanic action usually doesn’t extend beyond a few millimeters from the junction. Consequently, geometrical factors such as shape and size of the coupled metals have a relatively minor effect on galvanic corrosion in atmospheric conditions.
Marine atmospheres exhibit particularly significant galvanic action due to the high conductivity of seawater. Among the various forms of moisture that occur under atmospheric conditions, rain is especially effective in inducing galvanic corrosion. Galvanic corrosion rates during open exposure are notably higher than normal corrosion rates, while rates are similar under a rain shelter due to the differences in the electrolyte layer formed by rain versus moisture from condensation.
E3. Natural Waters
Natural waters, broadly categorized into seawater and freshwater (which includes river, lake, and underground waters), present distinctive differences as corrosion environments. One key distinction is the level of conductivity, with seawater having high conductivity due to its salt content, while freshwater typically has low conductivity. In the context of corrosion, natural waters are relatively homogeneous environments, with properties like seawater remaining consistent across time and geographic locations. This homogeneity affects the galvanic corrosion behavior observed in these waters.
Seawater, characterized by its high conductivity and uniformity, fosters long-range galvanic action that spreads uniformly over the entire surface of metallic structures. In contrast, galvanic effects in freshwater are generally less pronounced due to the lower conductivity.
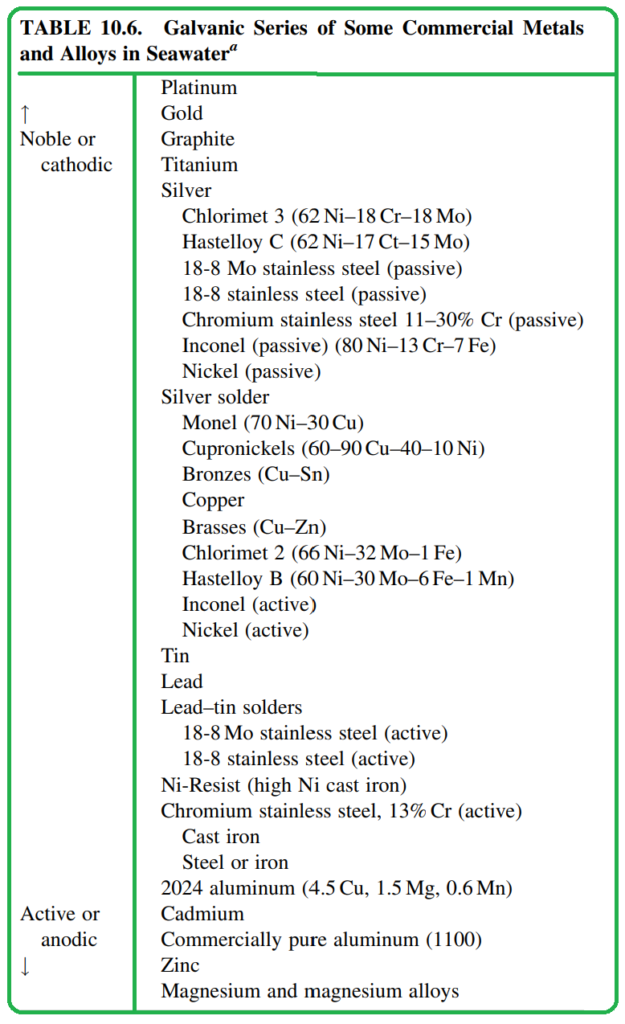
Table 10.6 provides a galvanic series for various commercial metals and alloys in seawater. It’s important to note that this galvanic series differs from the electromotive force (emf) series and is specific to seawater. Additionally, there are reported galvanic series for common alloy couples in flowing seawater.
Galvanic corrosion in seawater has been extensively studied, as indicated in Table 10.1. Table 10.7 presents data on the galvanic behavior of different bimetallic couples after extended exposure in both seawater and freshwater. Notably, galvanic action is much more pronounced in seawater compared to freshwater. In seawater, the corrosion rate of the anodic metal, such as zinc or steel, can be 5 to 12 times greater than in the uncoupled condition, whereas in freshwater, this increase is typically only 2 to 5 times.
Studies on Galvanic Actions of Miscellaneous Alloys In Various Environments
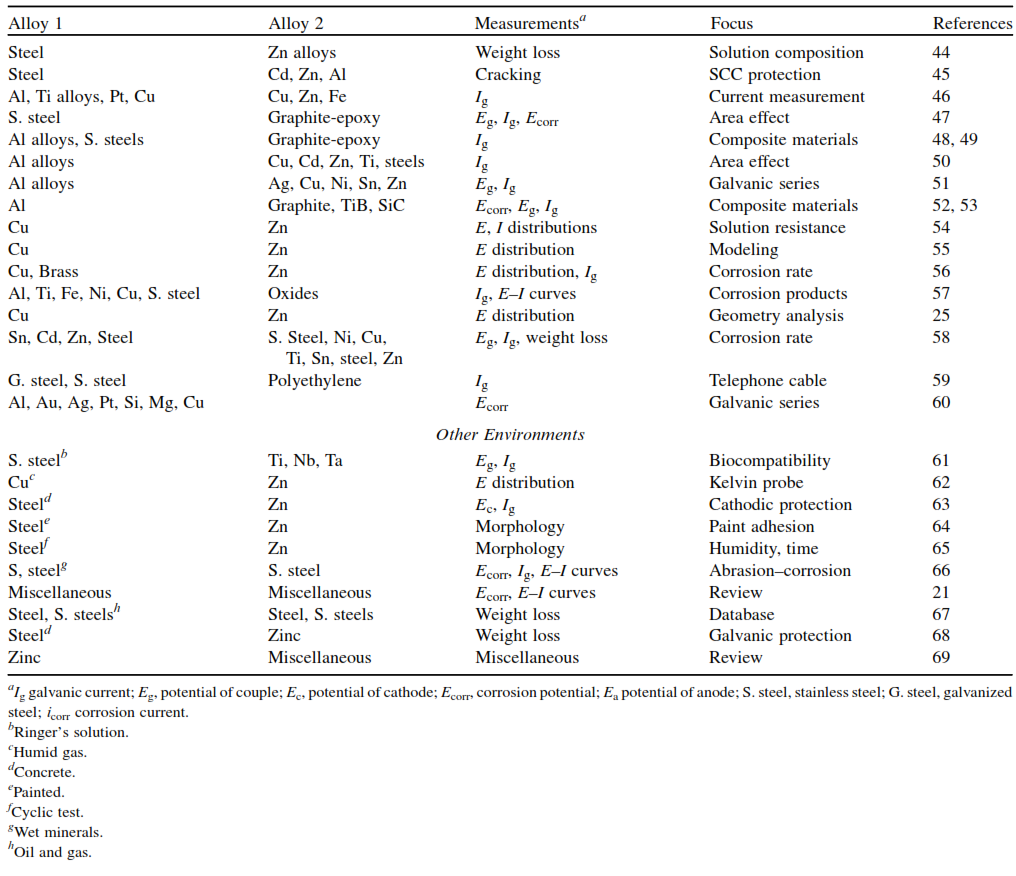
The data also highlight the significant influence of the relative sizes of the anode and cathode; changing the anode from a strip to a plate for the same bimetallic couple can result in 6 to 7 times more corrosion on the anode. Furthermore, Table 10.7 indicates that the corrosion of the cathodic member is generally reduced, to varying degrees, as a consequence of the galvanic corrosion of the anodic member.
F. POLARITY REVERSAL
Polarity reversal in galvanic couples is a phenomenon where the normal polarity between two metals changes over time under specific conditions. This phenomenon was first observed by Schikorr in 1939 in a zinc–steel couple immersed in hot supply water, where iron became anodic to zinc. This has been particularly problematic for galvanized steel hot water tanks. Since then, polarity reversal has been extensively studied and investigated.
The occurrence of polarity reversal is primarily linked to changes in the surface condition of at least one of the metals in the couple, often involving the formation of a passive film. The extent of passivity, the redox reactions present in the solution, and the overall stability of the system collectively determine the polarity and its evolution over time. For example, in a zinc–steel couple, the change in potential of the zinc electrode plays a significant role in causing polarity reversal, especially since the potential of the steel remains relatively constant over time in hot water environments. Notably, polarity reversal tends not to happen in distilled water at temperatures up to 65°C and in the absence of oxygen.
The rate and occurrence of polarity reversal can vary, ranging from minutes to several days. Factors such as temperature, dissolved ions, pH, and immersion time influence polarity reversal in a zinc–steel couple. The essential conditions for polarity reversal include the passivation of the zinc surface and the presence of certain reducing species, such as dissolved oxygen in water, which facilitates cathodic depolarization.
Polarity reversal has also been observed in aluminum–steel couples in natural environments, particularly when aluminum alloys are used as sacrificial anodes to protect steel. The mechanism is similar to that of the zinc–steel couple. However, aluminum tends to be naturally passivated by a thin oxide film in most environments. The potential of aluminum varies based on the extent of passivity, which can be influenced by specific ionic species in the environment. For instance, carbonate and bicarbonate ions promote passivity and lead to more noble potential values, while chloride ions have the opposite effect. To counter polarity reversal, sacrificial aluminum anodes are often alloyed with various elements.
The consequences of polarity reversal can be quite significant. In zinc–steel and aluminum–steel couples, where zinc and aluminum function as sacrificial anodes for steel protection, polarity reversal can lead to the loss of cathodic protection for the steel. This, in turn, results in galvanic corrosion of the steel and a shortened lifespan for structures made of steel.
G. GALVANIC COROSSION PREVENTIVE MEASURES
Preventing or minimizing galvanic corrosion is crucial to maintaining the integrity of metal structures and systems. While complete prevention may not always be practical, there are several measures and strategies that can be implemented to reduce the risk and extent of galvanic corrosion. Here are some practical approaches:
- Avoid Combinations of Dissimilar Metals: One of the most straightforward ways to prevent galvanic corrosion is to avoid using combinations of dissimilar metals that are far apart in the galvanic series relevant to the specific environment. By selecting metals with similar electrochemical properties, the potential for galvanic corrosion is reduced.
- Control Anode and Cathode Sizes: Avoid situations where the anode (the more reactive metal) is much smaller than the cathode (the less reactive metal). This size imbalance can lead to accelerated galvanic corrosion. Balancing the sizes of the coupled metals can help mitigate this effect.
- Isolate Metals from the Environment: Electrically separate or isolate the dissimilar metals from each other using insulating materials. Physical isolation can prevent the direct contact of metals, minimizing the galvanic effect. This approach is commonly used in electrical wiring and electronic devices.
- Environment Modification: Reduce the aggressiveness of the environment by adding corrosion inhibitors. These inhibitors can help create a less corrosive environment, slowing down the corrosion rate of metals. This is often employed in cooling systems and industrial processes.
- Cathodic Protection: Implement cathodic protection for the bimetallic couple. This involves connecting the metal to be protected to a rectifier or a sacrificial anode, which is a more reactive metal that corrodes sacrificially instead of the protected metal. This method is frequently used in pipelines, underground structures, and marine applications.
- Increase Solution Path Length: In situations where it’s feasible, increase the length of the solution path between the two coupled metals. This method is particularly effective in low-conductivity electrolytes like freshwaters. However, in highly conductive media such as seawater, the distance between metals has less impact due to the strong galvanic action.
It’s important to note that the choice of which approach or combination of approaches to use depends on the specific requirements and constraints of each application. Furthermore, addressing galvanic corrosion is most effective when considered early in the design and construction stages, as retrofitting solutions can be more challenging and less effective. Proper material selection, design considerations, and maintenance practices play a significant role in preventing or mitigating galvanic corrosion.
H. BENEFICIAL EFFECTS OF GALVANIC CORROSION
Galvanic corrosion can have beneficial effects when it leads to the protection of a more noble, cathodic metal or alloy by sacrificing a less noble, anodic metal. This phenomenon is widely utilized in various corrosion prevention methods:
- Sacrificial Anodes: Sacrificial anodes, typically made of metals like zinc, aluminum, or magnesium, are extensively used for corrosion protection in underwater and underground structures. These sacrificial anodes are connected to the metal to be protected (usually steel) and corrode sacrificially, effectively preventing the corrosion of the more critical metal. Different anode materials are chosen based on their electrochemical properties and suitability for specific environments.
- Galvanized Coatings: Galvanization is a common method where steel is coated with a layer of zinc. This zinc coating serves a dual purpose. It acts as a barrier layer, protecting the underlying steel, and also acts as a sacrificial anode. When discontinuities occur in the zinc coating, such as scratches or cuts, the exposed steel is protected by the galvanic action of the adjacent zinc. This combination of barrier and galvanic protection significantly enhances the corrosion resistance of steel.
- Effectiveness of Galvanic Protection: Galvanic protection can be highly effective, especially in environments where pollutants are present. In some cases, the reduction in the corrosion of the cathodic metal (e.g., steel) can be substantial. For example, galvanic protection reduced the corrosion of steel by factors of 3 times in rural atmospheres, 40 times in industrial atmospheres, and 300 times in seacoast industrial atmospheres, as compared to uncoupled conditions. Even though the anodic metal (e.g., zinc) experiences some corrosion, it is minimal compared to the protection it provides to the steel.
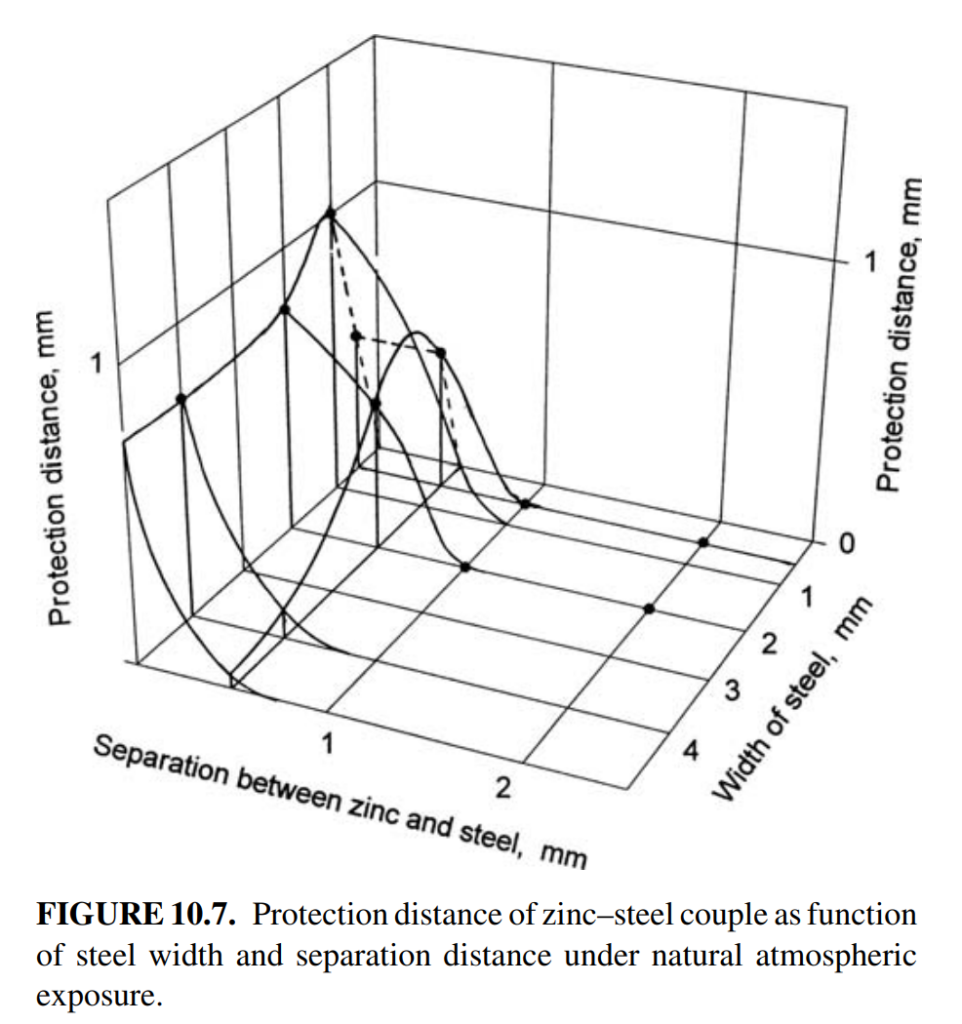
- Protection Distance: The protection distance of steel by a zinc coating in atmospheric environments is limited, typically extending only a few millimeters from the zinc coating. This limitation is due to the high resistance of thin-layer electrolytes formed in the atmosphere. However, the actual protected area, which includes regions under partial protection, is often larger than the immediate vicinity of the zinc coating.
- Figure 10.6. Figure 10.7 shows the protection distance as a function of separation distance and width of steel determined in an atmospheric environment.
Overall, galvanic corrosion can be harnessed as a cost-effective and efficient method for preventing the corrosion of critical metals and structures, particularly in harsh environments like marine or underground conditions. Proper selection of sacrificial anodes and coatings, along with maintenance practices, ensures the longevity and effectiveness of galvanic protection systems.
![FIGURE 10.6. Protection distance X as function of electrolyte thickness (t), steel width (W), and distance
between zinc and steel (D): (a) D ¼ 0; (b) D ¼ 5 mm [87]. (Copyright ASTM. Reprinted with permission.)](https://paktechpoint.com/wp-content/uploads/2023/08/image-193-1024x454.png)
I. FUNDAMENTAL CONSIDERATIONS
I1. Electrode Potential and Kirchhoff’s Law
The direction of galvanic current flow between two connected bare metals is determined by their actual electrode potentials in a corrosion environment. Specifically, the metal with the higher electrode potential (more positive, more noble, or more cathodic) becomes the cathode in the galvanic couple, while the other metal becomes the anode.
In real-world scenarios, the polarity of galvanic couples can often be neglected. However, it’s important to note that both the cathodic (Ec) and anodic (Ea) potentials are functions of the galvanic current (I). Consequently, when there is a current flow through the electrolyte, the potential difference between the two metals is not equal to the open-circuit cell potential.
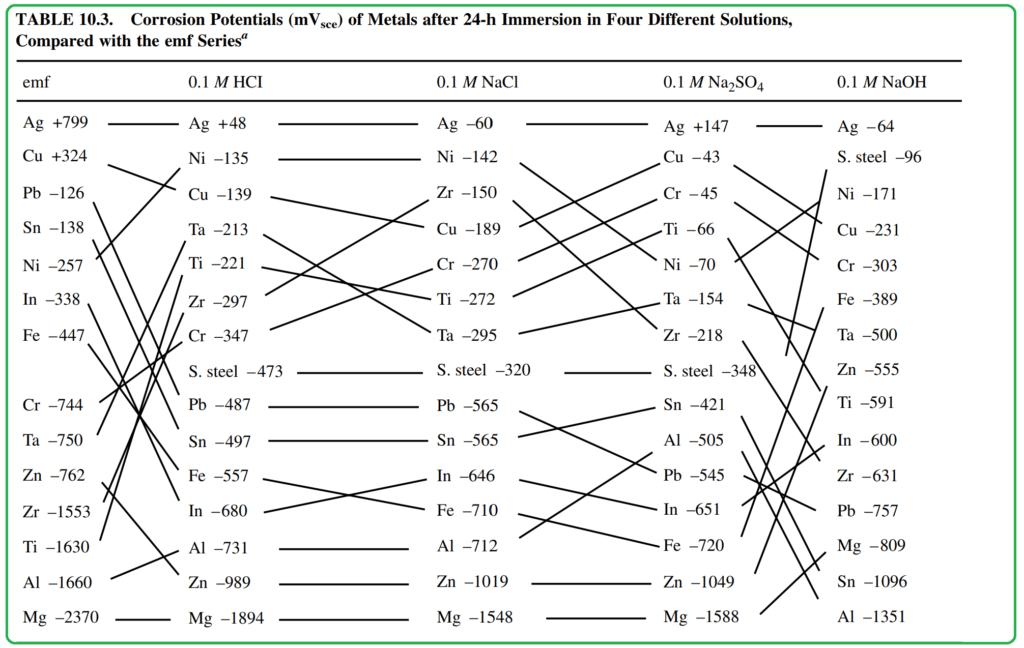
The actual position of each metal or alloy in a specific environment forms a galvanic series that typically differs from the thermodynamic reversible potential in the electromotive force (emf) series. This discrepancy arises because corrosion potentials are influenced by the reaction kinetics occurring at the metal-electrolyte interface. Thus, the galvanic series reflects the relative positions of metals in a specific environment and indicates the direction of galvanic current flow. However, it doesn’t provide information about the magnitude of the current or the corrosion rate, which depend on various other factors.
Kirchhoff’s second law is fundamental in understanding galvanic corrosion and relates the effective (polarized) potentials of the cathodic and anodic members (Ec and Ea) to the galvanic current (I) and the resistances within the galvanic circuit:
Ec – Ea = IRe + IRm
Here, Re represents the resistance of the electrolytic part of the galvanic circuit, Rm stands for the resistance of the metallic portion, Ec is the effective potential of the cathodic member, and Ea is the effective potential of the anodic member. Typically, the resistance of the metallic portion (Rm) is quite small and can be negligible in many cases.
More Explanation of Fundamentals Consideration of Galvanic Corrosion. Please Visit following article.