Erosion-corrosion is a destructive phenomenon that occurs when the combined action of both corrosion and mechanical wear (erosion) leads to material degradation in various industrial systems. This phenomenon is particularly prevalent in industries such as oil and gas, chemical processing, and power generation where fluids or slurries containing abrasive particles are transported at high velocities. Here are some key points about erosion-corrosion:
MECHANISM OF EROSION CORROSION
Following is the details of mechanisms of erosion, corrosion, and erosion-corrosion, particularly in the context of carbon steel in CO2 environments. It highlights the complex interactions between these processes and the various factors that influence them.
Mechanisms of Erosion:
- Erosion is primarily defined by the flow velocity of the fluid.
- At low velocities, scale formation occurs, resulting in low corrosion rates.
- At high erosivity (high velocity), uniform corrosion takes place at high rates.
- At intermediate velocities, pitting corrosion is often observed, with extremely high penetration rates.
- Erosion can cause material wastage through three primary mechanisms: chemical dissolution of the pipe, mechanical erosion due to fluid flow, and impingement of particles on the pipe wall.
Mechanisms of Corrosion:
- While corrosion mechanisms are well understood, CO2 corrosion in the oil and gas industry is complex and under ongoing investigation.
- CO2 can cause general and localized corrosion, and the type of corrosion varies depending on environmental conditions.
- CO2 can dissolve to form carbonic acid, bicarbonate, and carbonate anions, affecting the pH and buffering action.
- Iron carbonate is often the primary corrosion product.
- Factors such as CO2 partial pressure, temperature, and flow velocity influence corrosion rates.
Mechanisms of Erosion-Corrosion:
- Erosion-corrosion is a complex mechanism resulting from the interaction of electrochemical corrosion and mechanical erosion processes.
- The damage caused by erosion-corrosion is often greater than the sum of pure erosion and corrosion, indicating synergistic effects.
- The mechanism involves interactions between high-velocity corrosive mediums (e.g., gas-oil-water emulsions) causing cavitation and impingement and electrochemical reactions between the medium and exposed surfaces.
- Mass loss in erosion-corrosion can occur due to various mechanisms, including flow, impingement of suspended particles on pipe walls, electrochemical corrosion-enhanced erosion, or vice versa.
- Understanding erosion-corrosion mechanisms has led to the identification of different regimes, ranging from “erosion-dominated” to “corrosion-dominated,” depending on the relative intensity of erosion and corrosion processes.
In summary, erosion, corrosion, and erosion-corrosion are complex processes influenced by numerous factors, including flow dynamics, fluid properties, environmental conditions, and material properties. Understanding these mechanisms is crucial for effectively managing and mitigating material degradation in various industrial applications.
Synergism of Erosion-Corrosion
Synergistic effect of erosion and corrosion, particularly in the context of erosion-corrosion, which is a complex phenomenon where both mechanical and chemical processes contribute to material degradation. Following are points regarding the synergism of erosion-corrosion:
- Synergistic Nature: Erosion and corrosion have a synergistic effect on each other when they occur concurrently. In other words, the combined effect of erosion-corrosion is greater than the sum of its individual components (pure mechanical erosion and electrochemical corrosion).
- Complexity of Understanding: The mechanism of this synergism is not yet thoroughly understood due to its complexity. There are challenges in clearly separating the damage caused by erosion and corrosion, and this difficulty may arise from differences between laboratory-scale studies and real-world field conditions.
- Mathematical Representation: Synergism can be mathematically expressed as the difference between the material loss due to erosion-corrosion (T) and the sum of the material loss due to pure mechanical erosion (E) and electrochemical corrosion (C). This difference is represented by the synergistic term (S).
- T = E + C + S (Equation 1)
- S = T – (E + S) (Equation 2)
- Components of Synergism: The synergistic term (S) can be further divided into two components: ∆E (erosion-corrosion) and ∆C (corrosion enhanced erosion). In this way, S represents the combined interaction between corrosion and erosion processes.
- S = ∆E + ∆C (Equation 3)
- Influence on Material Properties: Synergistic effects during erosion-corrosion processes may alter material properties, such as hardness, as a result of surface damage. This property degradation can have significant implications for the resistance of the material to erosion.
- Evaluation through Experimental Program: To assess synergism, an experimental program typically includes three types of tests:
- Pure erosion tests to determine the rate of erosive wear.
- Pure corrosion tests to determine the rate of corrosive wear.
- Combined tests that simulate conditions in both erosion and corrosion environments to determine the total wear rate.
- Mechanisms of Synergism: Several mechanisms have been proposed to explain the magnification of the wear rate by synergism. One such mechanism suggests that fresh surfaces created during erosion are not immediately passivated by corrosion products, allowing them to corrode at an accelerated rate. Another mechanism highlights the degradation of material properties due to corrosion, reducing surface resistance to erosion.
In summary, erosion-corrosion involves a complex interplay between mechanical and chemical processes, resulting in synergistic effects that can significantly impact material degradation. Understanding and quantifying these effects are essential for designing materials and systems that are resistant to erosion-corrosion in practical applications.
MONITORING OF EROSION CORROSION
Monitoring of erosion corrosion, highlighting the challenges in conducting corrosion tests, the need to evaluate sand production in oil wells, and various methods and techniques used for measuring erosion corrosion rates. Here are the key points:
- Challenges in Corrosion Tests:
- Corrosion tests are difficult to replicate due to various factors, including surface/sample preparation and environmental conditions like flow velocity, pH, temperature, and concentration of corrosive species.
- Reproducibility of corrosion tests in different laboratories can vary between 55% to 75%.
- Importance of Sand Monitoring:
- Erosion corrosion may occur in the presence or absence of sand, but it is often believed that sand is present.
- As new sources of energy are being developed, oil sands will play an increasingly significant role in global oil production.
- Monitoring sand production in wells is essential, and this can be achieved using sand monitors and erosion or corrosion monitors.
- Electrochemical Methods:
- Electrochemical methods are widely used in both field and laboratory settings to measure corrosion and erosion-corrosion rates.
- Techniques include weight loss, Linear Polarization Resistance (LPR), Electrochemical Impedance Spectroscopy (EIS), Electrochemical Noise (EN), and externally monitored hydrogen permeation foils.
- LPR has been particularly useful in quickly identifying conditions where erosion-corrosion may be a serious threat.
- Laboratory Test Techniques:
- Several laboratory test techniques are used to simulate hydrodynamic conditions that materials experience during erosion-corrosion.
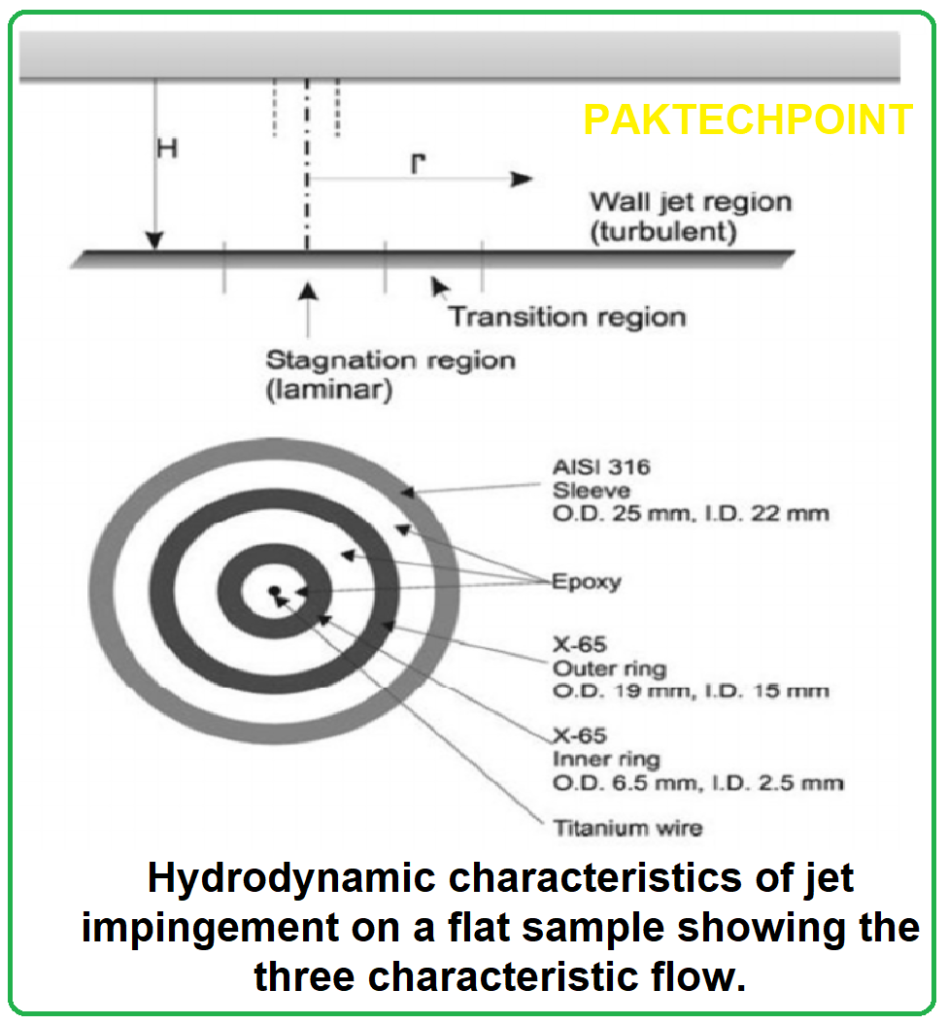
- Three primary methods are mentioned: Rotating Disk Electrode (RDE), Rotating Cylinder Electrode (RCE), and Submerged Impingement Jet system (SIJ).
- Each method has its advantages and shortcomings. RDE is preferred for laminar flow conditions, while RCE is suitable for investigating the entire flow regime from laminar to turbulent. SIJ is useful for studying flow-induced localized corrosion.
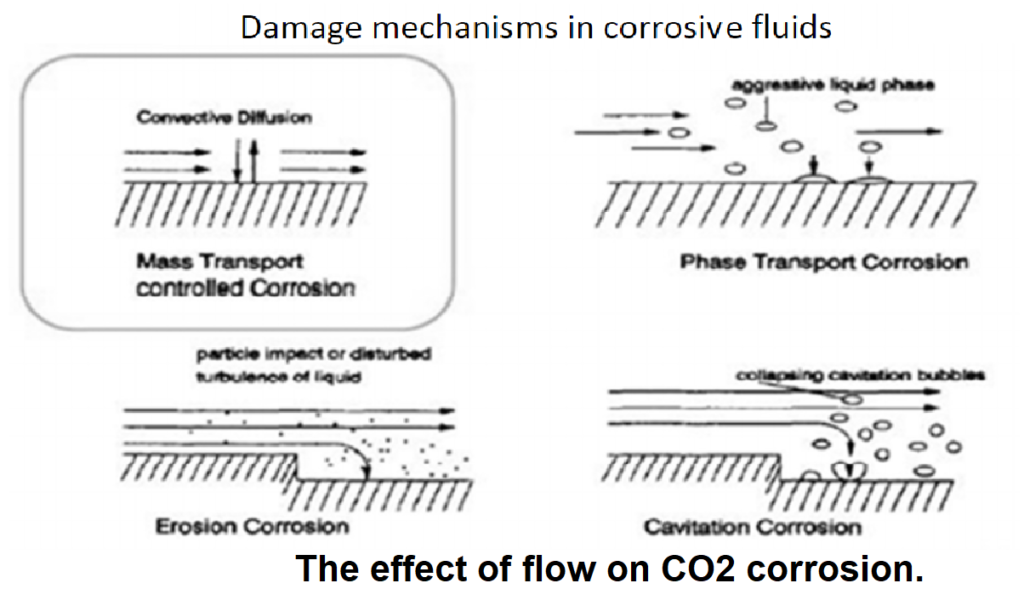
- Differentiating Corrosion Experiments and Electrochemical Measurements:
- The passage points out a distinction between corrosion experiments and electrochemical measurements.
- The depth of information obtained from flow-influenced electrochemical measurements is sometimes underestimated or ignored.
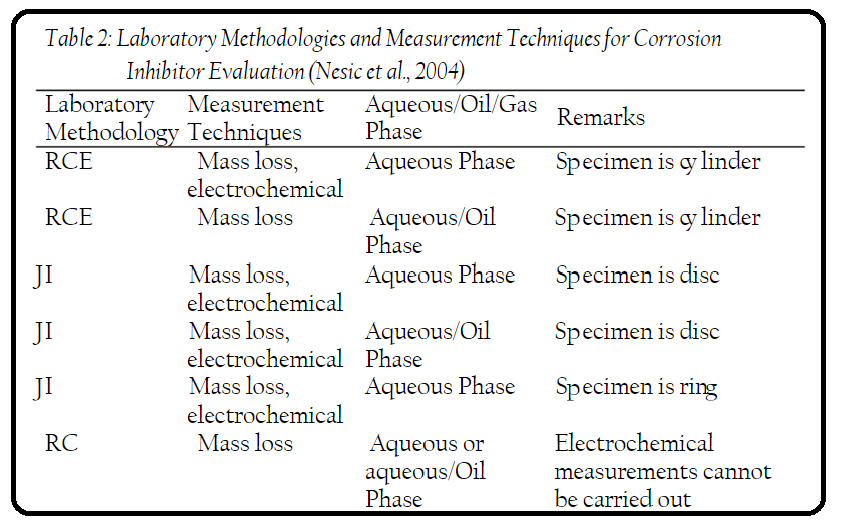
- Inhibitor Evaluation:
- Laboratory tests for inhibitor evaluation consist of two main components: laboratory methodology and measurement technique.
- Different combinations of these components are used for evaluating inhibitors for multiphase systems.
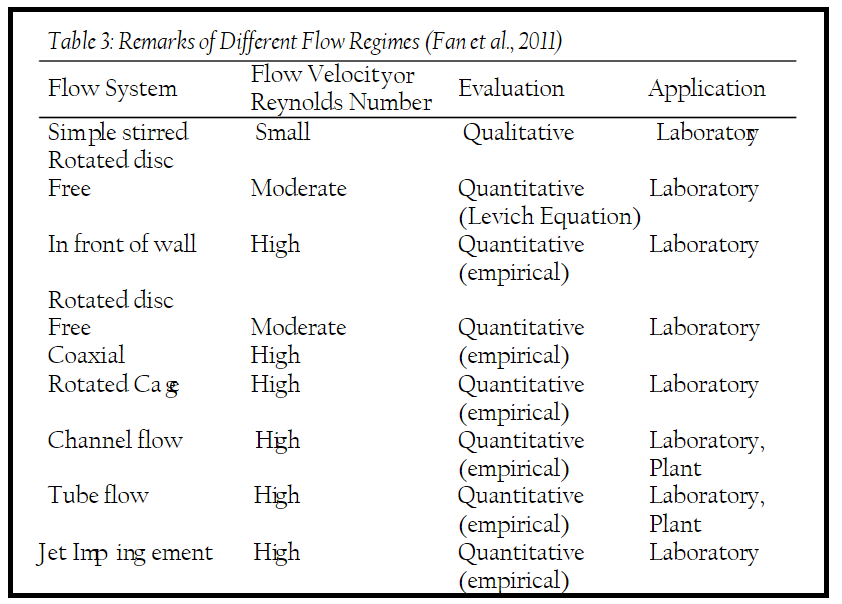
- Flow Systems and Experimental Tools:
- Tables are provided listing different flow systems and related experimental tools used in flow-related corrosion research and protection.
- These tables categorize the tools based on flow intensity, availability of equations for quantitative evaluation, and suitability for laboratory or field applications.
- Devices for Erosion-Corrosion Research:
- Various devices and methods, including RDE, RCE, impingement jet systems, slurry pots, rotating cages, slurry drums, and coriolis testers, are used for erosion-corrosion research.
- RDE and RCE are used to investigate the effects of hydrodynamic conditions on corrosion and erosion-corrosion, while impingement jet systems are suitable for studying mechanical impingement of solid particles.
In summary, monitoring and measuring erosion corrosion rates involve a combination of electrochemical techniques, laboratory test methodologies, and specialized equipment to simulate and evaluate the complex interactions between mechanical erosion and chemical corrosion processes. These methods are crucial for assessing and mitigating erosion-corrosion in various industrial applications.
MODELLING OF EROSION CORROSION
Modeling of erosion-corrosion, particularly in the terms of CO2 corrosion in the oil and gas industry.
- Role of Modeling: Modeling is a valuable tool for understanding degradation mechanisms, predicting damage levels, failure modes, and the likely locations of corrosion. These predictions can assist in developing practical guidelines for mitigating corrosion failures in the pipeline industry.
- Shift Towards Modeling: While a significant amount of erosion-corrosion research has traditionally relied on experimental methods, there is a current trend towards using modeling techniques as an additional investigative tool.
- Varieties of CO2 Corrosion Models: There are numerous prediction models for CO2 corrosion of carbon steel. Most of these models are semi-empirical or empirical, with only a few based on mechanistic descriptions of the underlying processes.
- Historical Development: The history of CO2 corrosion prediction models dates back to 1975 with the introduction of the de Waard and Milliams model. This model considered the impact of CO2 partial pressure and temperature on corrosion rates.
- Advantages and Limitations: Nyborg (2002) categorized CO2 corrosion prediction models into academic-based and industry-driven. He highlighted that major factors in CO2 corrosion include pH, protective films, oil wetting, presence or absence of H2S, top-of-line corrosion, and fluid dynamic parameters.
- Importance of Fluid Flow: Fluid flow parameters, particularly flow velocity, play a critical role in CO2 corrosion. However, some models have simplified fluid flow calculations, and only a few incorporate fluid flow considerations.
- Hydrodynamic Factors: Recent research has emphasized the importance of hydrodynamic factors in CO2 corrosion within the oil and gas industry. These factors have a significant influence on the corrosion process.
One significant parameter in erosion corrosion is the velocity of the impacting particles and the general form of the equation that relates erosion to velocity and it is given as:
ε = k. Vn f(θ)
where,
ε is the erosion rate; k is a constant and is a function of particles and targets, V is the velocity; n is the velocity exponent and θ is the impact angle in degrees.
Modeling is increasingly being used to predict and understand erosion-corrosion, with a focus on CO2 corrosion in the oil and gas industry. These models take into account various factors, including pH, protective films, oil wetting, presence of H2S, and fluid dynamics, particularly flow velocity. The incorporation of fluid flow considerations in modeling is recognized as an important aspect of accurately predicting CO2 corrosion rates.
Prevention and Control Strategies for Erosion-Corrosion
Prevention and control strategies for erosion-corrosion, particularly of the oil and gas industry. Here are the key points:
- Surface Modification Techniques: Erosion corrosion is primarily a surface phenomenon, and various surface modification techniques can be employed to improve corrosion and tribological (wear) properties. These techniques include cathodic protection, materials selection, design, coatings, and the use of inhibitors.
- Challenges with Ceramic Coatings: Some workers have used highly erosion-resistant ceramic coatings like TiN and CrN. However, these ceramic materials are brittle and can ultimately fail, leading to catastrophic consequences. They are expensive and typically reserved for critical applications.
- Design Considerations: At the design stage, it is advisable to remove flow barriers such as blind tees and sharp corners. The use of cathodic protection has also been proposed as an effective method to reduce material wastage significantly.
- Mitigation Strategies: In the industry, two primary mitigation strategies are commonly used: proper materials selection and the use of inhibitors.
- Materials Selection: Materials selection is a major challenge in dealing with erosion-corrosion, especially in the presence of sand erosion. Low carbon steel is a common choice for construction materials, but it can be improved through heat treatment and alloying. Materials selection options include corrosion-resistant alloys, coatings (including hard metals, cermets, and ceramics), and surface degradation control techniques such as the use of inhibitors.
- Inhibitors: Inhibitors play a crucial role in mitigating erosion-corrosion. They can reduce metal loss during impingement and slow down active corrosion. The efficiency of corrosion inhibitors depends on their ability to recognize and react with the corrosion agents present in the system, such as chloride, oxide, and carbonate. Inhibitors form a film on the metal surface, interfering with either the anodic or cathodic reaction. The effectiveness of inhibitors can vary depending on flow intensities, with critical flow intensities above which inhibitors lose efficiency. The type and concentration of inhibitors, as well as their chemical structure, influence their performance.
- Organic Inhibitors: Organic, adsorption-type corrosion inhibitors are commonly used in the oil and gas industry. They act on the metal surface and their effectiveness depends on factors such as the metal’s nature and surface charge, the aggressive electrolyte’s type, and the chemical structure of the inhibitor. Effective inhibitors should chemisorb onto the metal surface to form a protective barrier layer.
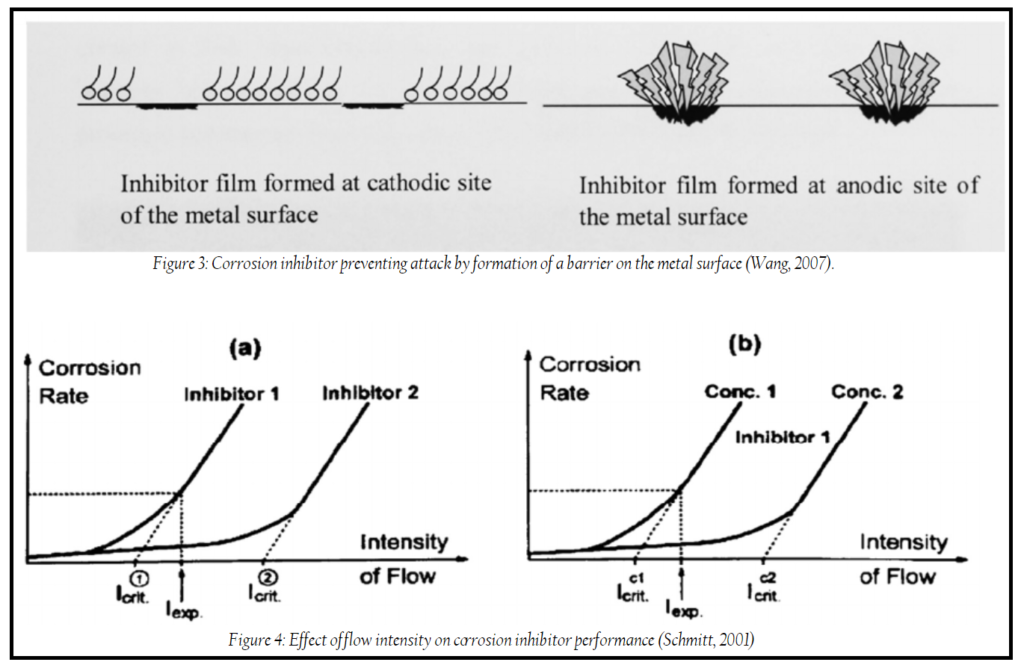
In summary, prevention and control of erosion-corrosion involve a combination of strategies, including materials selection, design modifications, and the use of inhibitors. The choice of approach depends on the specific conditions and requirements of the system in question.