This article is about a comprehensive overview of erosion-corrosion, its prevalence in the oil and gas industry, and its mechanisms.
What is Erosion Corrosion?
Erosion is described as the process of material removal from a solid surface due to the repeated application of mechanical forces. These forces can be caused by various factors, including solid particles, liquid droplets, or cavitation.
Erosion-corrosion is noted as one of the top five most prevalent forms of corrosion damage in the oil and gas sector, according to several surveys. This indicates its significance and impact on industry equipment.
In the context of carbon steel in CO2 environments, erosion-corrosion is characterized by the combined action of metal loss due to impingement and the corrosion of an active surface. The presence of suspended solids can significantly accelerate the erosion-corrosion rate compared to uniform corrosion.
Under suitable conditions in CO2 -saturated environments, FeCO3 scale is formed as a corrosion product. The specific corrosion regime varies with flow conditions:
- Low velocity results in FeCO3 scale formation with low metal loss rates.
- High velocity leads to sand abrasion, removal of the scale, and high metal loss rates.
- Intermediate velocities result in partial removal of the scale and localized pitting.
These erosion-corrosion regimes depend on the specific environmental conditions and the material of construction used in the equipment. Erosion in oil and gas production systems is primarily attributed to the presence of sand, along with liquid and gas. The mechanical forces generated by these solid particles can accelerate the erosion-corrosion process.
Erosion corrosion is defined as a type of accelerated corrosion that occurs following the removal of protective surface films. It is particularly severe when production velocities are high and involves rapid relative movement between flowing electrolytes (such as slurry) and metal components.
Impact on Materials: Erosion-corrosion can affect all metals to varying degrees. Given the corrosive nature of CO2 in oil and gas environments, it is essential to pay special attention to mechanisms like erosion-corrosion to prevent equipment degradation.
Factors that affect Erosion Corrosion.
Here we highlight various factors that can affect erosion corrosion, which is a complex corrosion process involving the mechanical removal of material from a solid surface due to the combined action of corrosion and mechanical forces. These factors can be broadly categorized into several groups:
1. Flow Dynamics.
- Fluid Velocity: Higher fluid velocities can intensify erosion corrosion by increasing the impact force of particles on the surface.
- Flow Regime: Changes in flow patterns, such as turbulence, can influence erosion corrosion rates.
- Component Geometry: The shape and configuration of the components, such as bends, tees, chokes, and joints, can affect the likelihood and severity of erosion corrosion.
2. Concentrations.
- Sand Characteristics: Parameters related to solid particles, including concentration, impact velocity, impact angle, number of particles hitting the surface, shape, sharpness, hardness, size distribution, and density, can significantly influence erosion corrosion.
3. Fluid Properties.
- Fluid Composition: The chemical composition of the fluid, including the presence of contaminants and corrosive species like oxygen, water, salts containing chloride, bicarbonate, and sulfate, can accelerate erosion corrosion.
- Density and Viscosity: These fluid properties affect the kinetic energy of particles in the fluid, which can impact their erosive potential.
4. Material Properties.
- Hardness: The hardness of the material plays a crucial role in its resistance to erosion corrosion.
- Microstructure: The microstructural characteristics of the material can influence its susceptibility to erosion corrosion.
5. Environment.
- pH: The acidity or alkalinity of the environment can affect the corrosion and erosion processes.
- Electrochemical Potential: The electrochemical conditions, including the potential, can have an impact on the corrosion and erosion rates.
- Chemical Composition: The presence of specific chemicals in the environment can either accelerate or mitigate erosion corrosion.
- Temperature and Pressure: These environmental factors can also influence the corrosion and erosion rates.
6. Component Geometry.
- The geometry of components, such as bends, tees, chokes, and joints, can affect the flow dynamics and the likelihood of erosion corrosion.
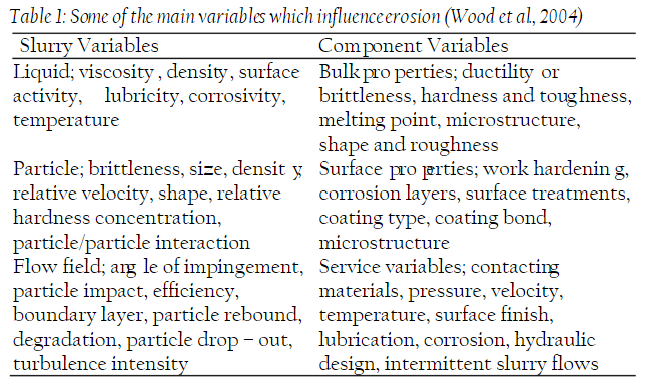
Understanding the interplay of these factors is crucial for assessing and mitigating erosion corrosion risks in various industrial applications. Comprehensive knowledge of these variables allows for the development of effective strategies to minimize the damage caused by erosion corrosion and ensure the longevity and safety of equipment and infrastructure.
Mechanism of Erosion Corrosion.
Erosion corrosion is a stealthy and destructive phenomenon that accelerates the deterioration of metals when they come into contact with corrosive fluids in motion. Unlike other forms of corrosion, erosion corrosion typically involves swift relative movement between the metal surface and the corrosive fluid, often accompanied by mechanical wear effects or abrasion.
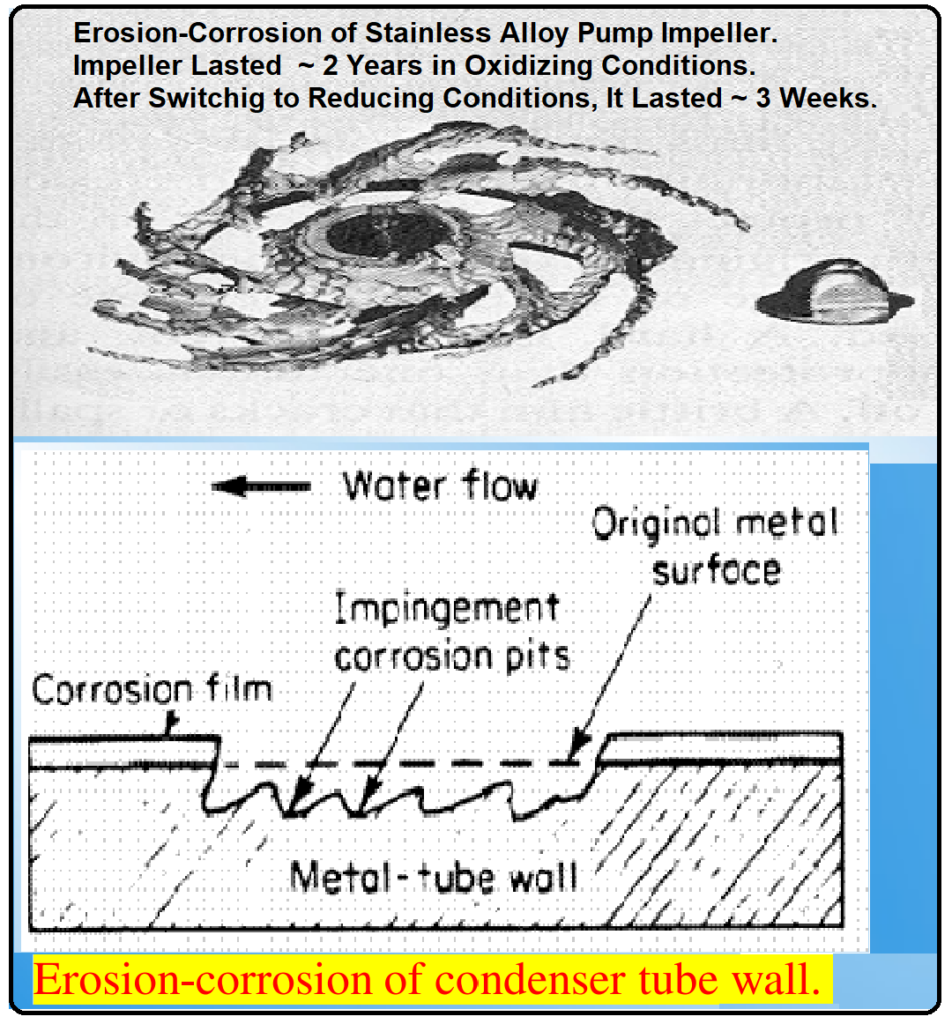
The Anatomy of Erosion Corrosion:
Erosion corrosion can manifest in two primary ways:
- Corrosion Product Removal: One mechanism involves the removal of corrosion products from the metal surface due to the intense fluid shear forces. These products essentially “slip off” the surface, exposing the bare metal beneath.
- Metal Ion Sweeping: Alternatively, metal ions, generated as a result of the corrosion process, may be carried away by the forceful fluid flow before they have a chance to deposit as corrosion products.
Erosion: The Mechanical Abrasion:
Erosion corrosion often involves mechanical abrasion caused by suspended particles within the fluid. Picture the effect of sandblasting or the erosion of turbine blades by the relentless onslaught of droplets. Here, it’s the straightforward wearing away of the metal surface due to physical abrasion.
Erosion Corrosion: Where Chemistry Meets Motion.
Erosion-corrosion takes the destructive potential of erosion a step further. In this scenario, the metal not only experiences mechanical wear but also undergoes chemical reactions in a corrosive environment. The result is a distinctive surface finish marked by grooves, waves, gullies, and holes, all aligned with the prevailing fluid flow pattern. This unique pattern, often described as “scalloping,” is a telltale sign of erosion-corrosion’s presence.
In conclusion, erosion corrosion is a formidable adversary for metals, particularly in situations involving the relentless movement of corrosive fluids. It combines the mechanical damage of erosion with the corrosive power of chemical reactions, leaving behind a distinctive and damaging surface landscape. Understanding and mitigating this phenomenon is crucial for preserving the integrity of metal components in various industrial settings.
Erosion-corrosion is a complex corrosion phenomenon that occurs in various environments and affects many metals and alloys, especially those that rely on protective surface films for corrosion resistance. Here’s a breakdown of the key points you’ve mentioned:
Susceptibility to Erosion-Corrosion:
- Many metals and alloys are susceptible to erosion-corrosion, especially those that depend on the formation of a protective surface film to resist corrosion. Examples of such materials include Aluminum (Al), Lead (Pb), Stainless Steel (SS), and Carbon Steel (CS).
Causes of Erosion-Corrosion:
Erosion-corrosion occurs when two main conditions are met:
- Erosion: This can be caused by factors such as high-velocity fluids, rapid changes in the direction of fluid flow, excessive turbulence, and the presence of suspended particles in the fluid.
- Corrosion: This happens when the rate of film formation on the metal’s surface is insufficient to protect it from dissolution and transfer to the bulk fluid. This can result from the erosive action or the nature of the corrosive environment.
Environments Where Erosion-Corrosion Occurs.
- Erosion-corrosion can be found in various environments, including:
- Aqueous Solutions: Such as in pipelines carrying water or aqueous chemical solutions.
- Gases: In high-velocity gas streams.
- Organic Liquids: In industries involving organic solvents and chemicals.
- Liquid Metal: In applications involving molten metals.
Impact of Suspended Solids.
If the fluid contains suspended solids, erosion-corrosion can be aggravated because these particles can accelerate the erosion process by physically impacting the metal’s surface.
Vulnerable Equipment.
Equipment that is most vulnerable to erosion-corrosion includes those subjected to:
- High-Velocity Fluids: Where the kinetic energy of the fluid can accelerate erosion.
- Rapid Changes in Fluid Direction: Which can create turbulence and increase erosive forces.
- Excessive Turbulence: Turbulent flow can promote both erosion and corrosion.
- Thin Boundary Layer: In equipment with a very thin boundary layer, there is high mass transfer, making the metal more susceptible to erosion-corrosion.
To mitigate erosion-corrosion, industries often use protective coatings, materials that are more resistant to this phenomenon, or design equipment to minimize high-velocity flows, turbulence, and rapid changes in fluid direction. Proper material selection and corrosion monitoring are also critical in preventing and managing erosion-corrosion issues.
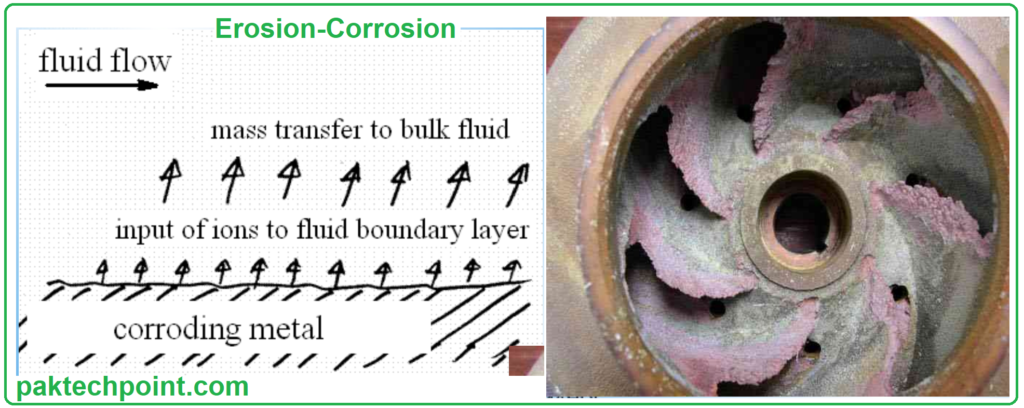
Vulnerable equipment includes:

Surface Film Effects.
Surface films play a crucial role in protecting materials from erosion-corrosion, and the quality and characteristics of these films significantly impact the material’s resistance to this phenomenon. Here are some key points related to the effects of surface films on erosion-corrosion:
- Importance of Protective Films: Corrosion-product films that form on the surface of metals are important for providing resistance to erosion-corrosion. These films act as barriers between the metal and the corrosive environment, helping to slow down the corrosion process.
- Desirable Film Characteristics: Films that are hard, dense, adherent, and continuous tend to offer better resistance to erosion-corrosion. These qualities ensure that the film can effectively protect the underlying metal from both mechanical and chemical attacks. However, it’s important that the film is not brittle and can withstand mechanical stress without easily fracturing.
- Effect of pH on Film Formation: The pH of the environment can have a significant impact on the formation and stability of protective films. For example, in the case of low-alloy steel, the scale formed on the surface can vary with pH. At pH values of 6 and 10, a protective film of Fe(OH)2/Fe(OH)3 forms, hindering the mass transport of oxygen and ionic species. This can contribute to the resistance of the material to erosion-corrosion.
- Lead Sulfate Film Example: Lead (Pb) can form a protective lead sulfate (PbSO4) film in dilute sulfuric acid (H2SO4) under stagnant conditions. However, this film might not provide sufficient protection under rapidly moving conditions where mechanical forces can remove or disrupt the film.
- Titanium (Ti) Resistance: Titanium exhibits good resistance to erosion-corrosion in various environments, including seawater, chloride (Cl-) solutions, nitric acid (HNO3), and others. This resistance is attributed to the formation and stability of titanium dioxide (TiO2) films on the surface of titanium. These films act as a barrier, protecting the underlying metal from both mechanical and chemical attacks.
- Copper (Cu) Alloys: The behavior of copper alloys in erosion-corrosion scenarios can be complex. The formation of protective films on copper alloys can depend on factors like composition, exposure conditions, and the specific corrosive environment.
In summary, protective surface films are critical for providing resistance to erosion-corrosion. The characteristics of these films, their formation, and their stability in different environments play a significant role in determining the material’s susceptibility to this type of corrosion.
Prevention of Erosion Corrosion.
Preventing erosion-corrosion is crucial to maintaining the integrity and longevity of equipment and components exposed to corrosive and erosive environments. Here are several strategies for preventing erosion-corrosion:
1. Design Considerations:
- Avoid Impingement Geometries: Design equipment and piping systems to minimize areas where high-velocity fluids directly impact the surface. This can reduce the erosive effects of the fluid.
- Control High Velocities: Minimize or control high fluid velocities, especially in areas where erosion-corrosion is likely to occur. This can be achieved through proper design, including the use of flow control devices.
- Turbulence Reduction: Design components and systems to minimize turbulence, which can accelerate both erosion and corrosion. Smooth flow paths and gradual changes in direction can help reduce turbulence.
2. Chemical Control.
- Adjust pH: In some cases, adjusting the pH of the fluid can help control the formation of protective films. Maintaining a pH level above a certain threshold can promote the formation of protective films that resist corrosion.
- Oxygen Control: In systems where oxygen promotes corrosion, such as in steam supply systems with carbon steel (CS) or low-alloy steel, reducing oxygen levels or using corrosion inhibitors can be effective.
- Use of Corrosion Inhibitors: Depending on the specific environment, the addition of corrosion inhibitors can help mitigate erosion-corrosion. For example, using morpholine instead of ammonia (NH3) in steam supply systems can be effective in preventing corrosion.
3. Material Selection.
- Use Corrosion-Resistant Materials: Choose materials that are inherently more resistant to corrosion and erosion. For instance, using chromium-containing stainless steels (e.g., 316 stainless steel) can provide better resistance in certain environments.
4. Coatings and Surface Treatments.
- Apply Protective Coatings: Utilize hard and corrosion-resistant coatings on the surface of components. These coatings can act as a barrier, protecting the underlying material from erosive and corrosive agents. Examples include ceramic coatings, polymer coatings, and thermal spray coatings.
- Surface Treatments: Treat the material surface to enhance its resistance to erosion-corrosion. This may involve processes like nitriding or carburizing to harden the surface layer.
5. Regular Inspection and Maintenance.
- Implement a regular inspection and maintenance program to detect erosion-corrosion damage early. This allows for timely repairs or replacements to prevent further degradation.
6. Fluid Flow Control.
- Use flow control devices, such as baffles, diffusers, or flow restrictors, to manage fluid flow patterns and reduce the impact of high-velocity flows on critical components.
7. Monitoring and Testing.
- Continuously monitor and test the condition of components exposed to erosion-corrosion-prone environments. This can include measuring corrosion rates, film formation, and the effectiveness of corrosion inhibitors.
Preventing erosion-corrosion often requires a combination of these strategies, tailored to the specific conditions and materials involved in a given application. A comprehensive approach that includes proper design, material selection, and maintenance practices is essential for effectively mitigating the risks associated with erosion-corrosion.
Cavitation Damage.
Cavitation damage is a phenomenon that can have effects similar to erosion-corrosion (E-C), but it occurs due to a different mechanism. In both cavitation damage and erosion-corrosion, there is mechanical removal of protective layers or material from the surface, resulting in surface degradation. Here are some key points about cavitation damage:
- Mechanism: Cavitation damage occurs when vapor bubbles form and then rapidly collapse within a fluid due to high-speed pressure fluctuations. The collapsing bubbles generate intense shock waves and microjets that can create localized areas of extremely high pressure, often exceeding 60,000 pounds per square inch (psi). These high-pressure events result in the mechanical removal of material from the surface.
- Surface Attack: Cavitation damage typically appears on the surface as closely spaced pitting or crater-like features. These pits are the result of the repeated collapse of vapor bubbles, which causes the removal of material from the metal surface.
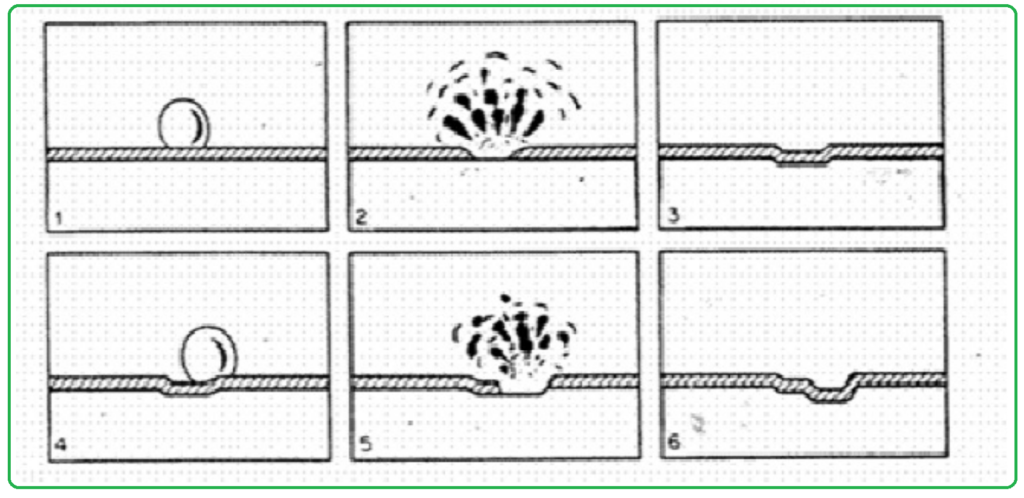
Fretting Corrosion.
Fretting corrosion is a form of localized corrosion that occurs due to the repetitive, low-amplitude rubbing or relative motion between two surfaces. It is similar to erosion-corrosion (E-C) in that it involves both mechanical action and corrosion, but the mechanism of mechanical action is different. There are two main theories explaining the mechanism of fretting corrosion, but they lead to the same overall result – surface damage and corrosion at the contact points. Here’s an overview of fretting corrosion and methods for its prevention:
1. Fretting Corrosion Mechanisms.
- Adhesive Wear Theory: This theory suggests that fretting corrosion occurs due to the adhesion and detachment of small wear particles at the interface of two surfaces. The repeated cycling of these particles can remove the protective oxide layer on the metal surfaces, exposing them to corrosion.
- Abrasive Wear Theory: In this theory, fretting corrosion is attributed to the abrasive wear of the surfaces in contact. As the surfaces rub against each other, they create abrasive debris. This debris can act as abrasive agents that remove the protective films, leading to corrosion.
2. Prevention of Fretting Corrosion.
Preventing fretting corrosion involves strategies aimed at reducing the mechanical action and protecting the surfaces from corrosion. Here are some common methods:
- Lubrication: Proper lubrication can reduce the friction and wear at the contact points, thereby minimizing fretting corrosion. Lubricants act as a barrier between the surfaces and help prevent metal-to-metal contact.
- Avoid Relative Motion: In some cases, it may be possible to eliminate or reduce relative motion by adding packing materials or spacers between the contacting surfaces. This reduces the risk of fretting corrosion by minimizing the contact and rubbing between components.
- Increase Relative Motion: Paradoxically, increasing the relative motion between two components can sometimes reduce the severity of fretting corrosion. This is because increased motion can prevent the formation of protective oxide layers or reduce the contact forces.
- Material Selection: Choosing materials that are more resistant to fretting corrosion can be effective. For example, selecting harder materials for one or both components can reduce wear and the tendency for fretting corrosion to occur.
- Surface Treatments: Applying coatings or surface treatments that enhance the wear resistance of the materials can help prevent fretting corrosion. These coatings can act as a sacrificial layer, protecting the underlying material.
- Design Modifications: Redesigning components to reduce the likelihood of fretting corrosion is another preventive measure. This may involve changes in the geometry or surface finish of the components.
- Regular Inspection and Maintenance: Implementing a maintenance program that includes regular inspection of components susceptible to fretting corrosion can help detect and address issues before they lead to significant damage.
Fretting corrosion is a complex phenomenon that involves both mechanical wear and corrosion. Preventive measures aim to reduce mechanical action, protect surfaces from corrosion, and select materials and designs that are less susceptible to this type of damage. The specific prevention strategy may vary depending on the application and the severity of the fretting corrosion.