Hydrogen-induced cracking, often referred to as hydrogen embrittlement, is a chemical phenomenon that leads to the fracture of metal alloys. This unique mode of mechanical failure primarily affects low-alloy steels and high-hardness steel grades, including materials like titanium (Ti). Components can become more susceptible to hydrogen-induced cracking due to the introduction of excessive hydrogen during manufacturing or finishing processes.

What is Hydrogen Induced Cracking (HIC)?
Hydrogen Induced Cracking (HIC) is a form of corrosion that occurs in carbon steel and certain low-alloy steel materials exposed to hydrogen-containing environments, especially in industries like oil and gas, petrochemicals, and refining. It is a serious and potentially catastrophic issue that can compromise the integrity of equipment and structures. Here are the key points about hydrogen-induced cracking:
- Formation of Atomic Hydrogen: HIC starts with the absorption of atomic hydrogen (H) into the steel material. This hydrogen can come from various sources, such as corrosion reactions, chemical processes, or as a byproduct of electrochemical reactions.
- Diffusion of Hydrogen: Once atomic hydrogen is absorbed into the steel, it can diffuse through the metal lattice. Hydrogen atoms are small and mobile, allowing them to penetrate the steel’s microstructure.
- Hydrogen Accumulation: As hydrogen atoms diffuse through the steel, they can accumulate at microstructural defects, such as grain boundaries, inclusions, and other imperfections. These areas become hydrogen traps.
- Hydrogen-Induced Cracks: The accumulated hydrogen atoms can cause localized stress within the material. When the stress exceeds a critical level, it can lead to the initiation and propagation of cracks. These cracks are known as hydrogen-induced cracks or hydrogen blisters.
- Microstructure Role: The microstructure of the steel plays a significant role in HIC susceptibility. Coarse-grained materials, materials with high levels of inclusions, and materials with low toughness are more susceptible to HIC.
- Environmental Factors: HIC is often associated with environments that contain hydrogen sulfide (H2S) gas, which is commonly found in the oil and gas industry. HIC can also occur in other hydrogen-containing environments.
- Prevention and Mitigation: Preventing HIC involves using materials with improved resistance to hydrogen embrittlement, proper material selection, and design considerations. Additionally, control measures such as reducing the H2S content, maintaining proper pH levels, and implementing corrosion inhibitors can help mitigate the risk of HIC.
- Testing and Inspection: Non-destructive testing (NDT) methods, such as ultrasonic testing and magnetic particle inspection, can be used to detect hydrogen-induced cracks. Regular inspections are crucial to identify and address HIC before it leads to equipment failure.
- Standards and Codes: Various industry standards and codes, such as NACE MR0175/ISO 15156, provide guidelines for materials selection and prevention of hydrogen-induced cracking in specific environments.
HIC is a complex issue that requires careful consideration of material properties, environmental factors, and operational conditions. Effective prevention and mitigation strategies are essential to ensure the integrity and safety of equipment and infrastructure in industries where HIC is a potential concern.
Hydrogen Induced Cracking (HIC) Process
The most significant concern associated with hydrogen-induced cracking is the gradual diffusion of hydrogen atoms into a component’s structure over its service life. This is an ongoing issue in industries dealing with sour service and environments containing wet hydrogen sulfide (H2S).
Let’s explain deeper into the mechanics of how hydrogen-induced cracking occurs:
Hydrogen Saturation:
This phenomenon begins when the crystal lattice structure of a metal alloy becomes saturated with diffusing hydrogen atoms. The rate of saturation tends to be higher at elevated temperatures due to the relationship between hydrogen solubility and temperature. As the temperature rises, hydrogen atoms diffuse into the metal, and some may combine to form larger hydrogen molecules.
Accumulation:
These larger hydrogen molecules can accumulate within tiny voids or pockets within the metal’s structure. This accumulation increases the internal pressure within the component.
Impact on Properties:
The presence of hydrogen within the metal lattice has adverse effects on its mechanical properties. It diminishes ductility and tensile strength, making the material more susceptible to fractures.
Crack Initiation:
The localized flaws caused by hydrogen-induced cracking can initiate cracks within the material. These cracks may propagate through the metal’s surface, ultimately leading to failure.
Hydrogen-induced cracking is a critical concern in various industries, particularly those involved in processing or containment facilities dealing with hydrogen and hydrogen sulfide. It is a complex form of corrosion that can result in costly equipment failures and pose health risks to personnel exposed to hydrogen sulfides.
Materials Susceptibility to Hydrogen Embrittlement
Hydrogen embrittlement is a phenomenon where hydrogen atoms accumulate in metal structures, causing them to become brittle and prone to fracture. This can affect various metals, including steel, aluminum, and titanium. Some metals are more susceptible to this than others.
Steels
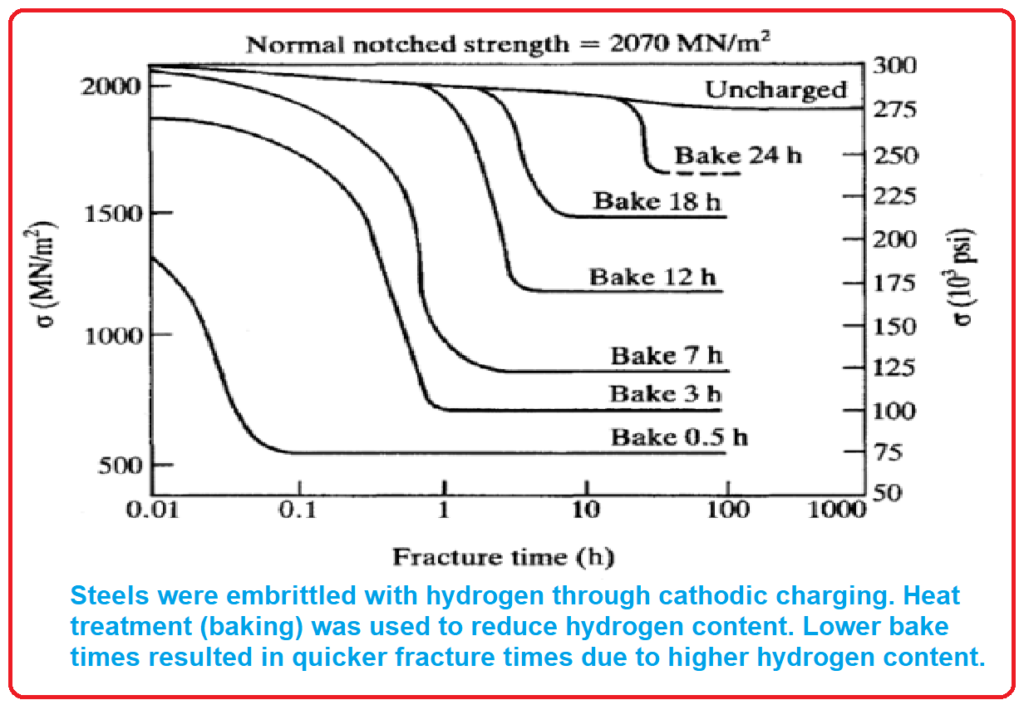
- Hydrogen Exposure: Steels can become embrittled when exposed to hydrogen, especially through cathodic charging.
- Heat Treatment: Heat treatment, also known as baking, can reduce the hydrogen content in steels.
- Strength Impact: Steels with low ultimate tensile strength (less than 1000 MPa) or low hardness (less than HRC 32) are generally not susceptible to hydrogen embrittlement.
- High-Strength Steels: As steel strength increases, the risk of hydrogen embrittlement also rises. High-strength steels with hardness above HRC 32 can experience early hydrogen cracking and long-term failures due to hydrogen accumulation.
- Quality Control: Steels with hardness in the range of HRC 32-36 and above should undergo quality checks to ensure they are not susceptible to embrittlement.
- Fracture Toughness Testing: Testing embrittled steel’s fracture toughness is complex, requiring very cold conditions to prevent hydrogen from diffusing away.
Copper
- Hot Hydrogen Exposure: Copper alloys with oxygen can become embrittled when exposed to hot hydrogen.
- Hydrogen Diffusion: Hydrogen diffuses through copper and reacts with inclusions, forming metallic copper atoms and water bubbles at grain boundaries.
- Steam Embrittlement: The process forces grains apart, known as steam embrittlement, due to the formation of water inside the copper lattice.
Vanadium, Nickel, and Titanium Alloys
- Hydrogen Solubility: Alloys containing vanadium, nickel, and titanium have high hydrogen solubility, allowing them to absorb significant amounts of hydrogen.
- Hydride Formation: Hydride formation can occur, causing irregular volume expansion and reduced ductility due to the fragility of metallic hydrides.
- Application Concerns: This is important when selecting alloys for use in applications like hydrogen separation membranes.
In conclusion, hydrogen embrittlement affects various metals differently, and factors such as material strength, exposure conditions, and hydrogen solubility play a role in susceptibility. Proper material selection, quality control, and understanding of hydrogen-related risks are crucial in preventing embrittlement-induced failures.
How to prevent hydrogen-induced cracking?
Preventing hydrogen-induced cracking (HIC) is essential to ensure the integrity and safety of metal structures, particularly in industries where exposure to hydrogen is common. Here are some key measures to prevent HIC:
1. Material Selection:
- Choose materials that are less susceptible to HIC. For example, use materials with lower hardness or higher ductility when hydrogen exposure is expected.
2. Proper Heat Treatment:
- Heat treatment processes, such as baking or stress relieving, can help reduce the hydrogen content in metals after they have been exposed to hydrogen during manufacturing or service.
3. Avoid Hydrogen Exposure:
- Minimize exposure to hydrogen during manufacturing and welding processes. Use protective atmospheres or barriers to prevent hydrogen from infiltrating metal surfaces.
4. Cathodic Protection:
- Implement cathodic protection systems in environments where metals are exposed to corrosive substances or hydrogen. These systems help control the electrochemical reactions that can lead to HIC.
5. Monitoring and Inspection:
- Regularly inspect metal components for signs of HIC, such as cracks or embrittlement. Non-destructive testing (NDT) methods like ultrasonic testing can be effective for detecting surface cracks.
6. Hydrogen Removal:
- Implement procedures to remove or vent hydrogen from enclosed spaces or equipment to prevent its accumulation.
7. Material Compatibility:
- Ensure that materials used in contact with hydrogen are compatible and resistant to embrittlement. This includes gaskets, seals, and coatings.
8. Temperature Control:
- Maintain proper operating temperatures, as higher temperatures can increase the solubility and diffusion of hydrogen in metals.
9. Corrosion Control:
- Implement corrosion control measures to reduce the formation of hydrogen in corrosive environments.
10. Training and Education:
– Train personnel working with hydrogen or in industries susceptible to HIC on best practices, safety protocols, and the risks associated with hydrogen exposure.
11. Material Testing:
– Prior to deployment, test materials in hydrogen-rich environments to assess their susceptibility to HIC. Use materials that demonstrate resistance to embrittlement.
12. Research and Development:
– Continuously invest in research and development to identify and develop materials and techniques that are more resistant to HIC.
Preventing hydrogen-induced cracking requires a combination of material selection, process control, monitoring, and ongoing maintenance. It’s essential to have a comprehensive strategy in place to mitigate the risks associated with HIC effectively. Additionally, staying updated with industry standards and guidelines is crucial to ensuring the safety and integrity of metal structures in hydrogen-rich environments.