The capacity of VRLA (Valve-Regulated Lead-Acid) batteries is influenced by several key factors. Firstly, the discharge rate plays a crucial role, with capacity decreasing as the discharge rate increases, largely due to the Peukert effect, which leads to reduced efficiency at higher discharge rates.
Ambient temperature also has a substantial impact, with capacity decreasing as temperatures drop; this effect is quantified by the temperature coefficient of capacity. Additionally, the depth of discharge, or how much the battery is discharged, can affect capacity, as deeper discharges may result in reduced capacity over time. Properly setting charging and discharging termination voltages is essential, as improper voltages can lead to capacity loss.
The battery’s design and quality, including its electrodes and separators, also influence capacity. Finally, maintenance practices, charging methods, and discharge management all contribute to the long-term capacity of VRLA batteries. Collectively, these factors determine the effective capacity of VRLA batteries under specific operating conditions.
What are Factors Effect VRLA Battery Capacity?
1. How Discharge rate affecting the battery capacity?
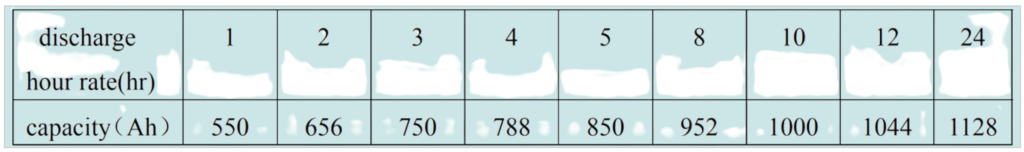
The relationship between discharge rate and battery capacity in lead-acid batteries is inversely proportional. As the discharge rate increases, the battery’s capacity decreases. Capacity is typically specified with a discharge hour rate, which indicates the rate at which the battery is discharged. The higher the discharge rate (i.e., the shorter the discharge time), the lower the effective capacity of the battery.
For example, a 1000AH (ampere-hour) battery will have different capacities when discharged at different discharge hour rates. At higher discharge rates, the battery’s capacity will appear to be lower than its rated 1000AH capacity due to the Peukert effect, which describes how a battery’s capacity decreases at higher discharge rates.
This relationship is important to consider when sizing and using lead-acid batteries for specific applications. It means that if you discharge a lead-acid battery at a high rate, you’ll get less total energy from the battery compared to discharging it at a lower, more gradual rate. Properly understanding and accounting for this relationship is crucial for ensuring that a battery meets the energy requirements of a given application.
2. Reasons for the capacity decline in high rate discharge
The capacity decline in high-rate discharge of lead-acid batteries can be attributed to several factors:
- Non-Uniform Current Distribution: At higher discharge rates, the discharge current becomes denser and less evenly distributed across the electrode’s surface. This uneven distribution results in a higher concentration of current on the nearest surface of the electrode.
- Formation of Lead Sulfate (PbSO4): Due to the uneven current distribution, lead sulfate (PbSO4) forms primarily on the outermost surface of the electrode. Lead sulfate has a larger volume compared to the original lead dioxide (PbO2) and lead (Pb) materials in the electrode.
- Electrode Porosity Clogging: The formation of lead sulfate in larger volumes can clog the porous structure of the electrode. This clogging restricts the flow of electrolyte to the reaction sites within the electrode, limiting the availability of reactants for the electrochemical reactions.
- Incomplete Utilization of Electrode Material: As the electrolyte supply becomes insufficient due to clogging, the electrode material inside the electrode cannot be fully utilized for the electrochemical reactions. This incomplete utilization of the active material results in a reduced capacity under high-rate discharge conditions.
The capacity decline during high-rate discharge in lead-acid batteries is primarily due to non-uniform current distribution, which leads to the formation of lead sulfate on the electrode’s surface and the subsequent clogging of the electrode’s porous structure. This restricts the flow of electrolyte and limits the full utilization of the electrode material, resulting in reduced battery capacity.
3. Relationship between discharge current and electrode effect
The relationship between discharge current and electrode effectiveness in lead-acid batteries can be summarized as follows:
- Effect of Discharge Current on Depth of Active Substance:
- In large current discharges, the depth within which the active substance is effectively utilized becomes limited. As the discharge current increases, this depth effect decreases.
- Higher current discharges result in shallower utilization of the active substance within the electrode.
- Impact on Battery Capacity:
- The degree of utilization of the active substance within the electrode is reduced as the discharge current increases.
- Reduced utilization of the active substance leads to a smaller capacity for the battery.
- At low current densities (i ≤ 100A/m2), the depth effect of the active substance is relatively deep (3 × 10-3m to 5 × 10-3m), allowing the full utilization of the inner porous structure of the electrode.
- At high current densities (i ≥ 200A/m2), the depth effect of the active substance sharply declines (to about 0.12 × 10-3), limiting its utilization depth.
- Diffusion as a Capacity-Limiting Factor:
- In high current discharge conditions with a low depth effect of the active material, diffusion becomes the primary factor limiting the battery’s capacity.
- Diffusion limitations can hinder the movement of reactants within the electrode, reducing the battery’s overall performance.
- Voltage Drop and Capacity:
- In large current discharges, the presence of polarization and internal resistance in the battery causes the terminal voltage to drop significantly.
- A rapid decrease in terminal voltage affects the battery’s capacity and overall performance.
High discharge currents in lead-acid batteries result in limited depth utilization of the active substance within the electrode. This reduced depth effect negatively impacts the battery’s capacity. Additionally, the presence of polarization and internal resistance at high currents leads to a rapid drop in terminal voltage, further affecting capacity and overall battery performance.
4. The impact of temperature on battery capacity
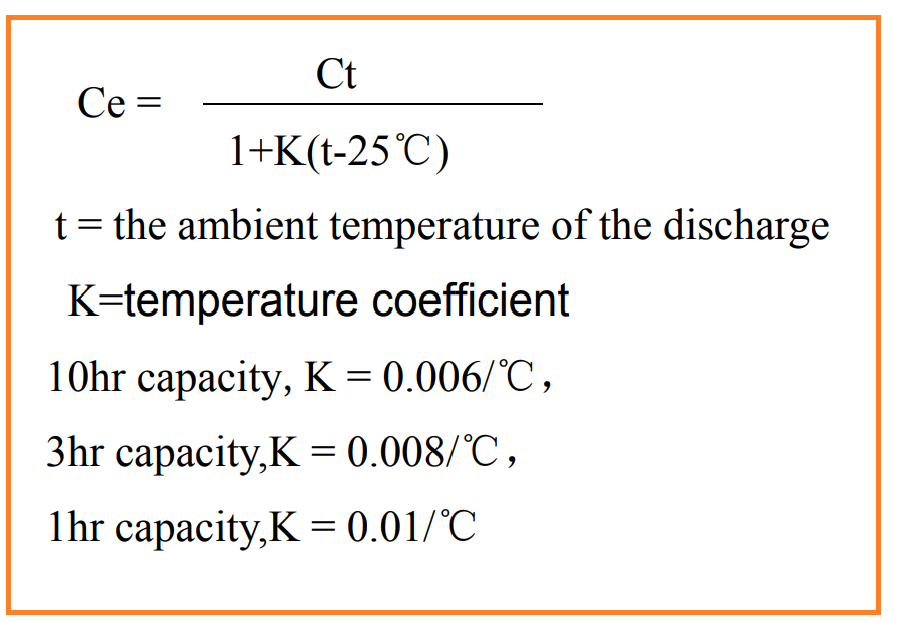
The impact of temperature on battery capacity is significant. Generally, as the ambient temperature decreases, the battery’s capacity also decreases. This relationship is characterized by the temperature coefficient of capacity, which quantifies how much the battery’s capacity changes with a 1℃ change in ambient temperature.
National standards often require the conversion of the actual measured capacity to a standardized reference temperature, typically 25℃. This conversion is done using a specific formula to determine the battery’s capacity at the reference temperature.
Temperature plays a crucial role in affecting battery capacity, and adjustments may be necessary to account for temperature variations when measuring and comparing battery performance.
5. Calculation of VRLA battery capacity
The calculation of VRLA (Valve-Regulated Lead-Acid) battery capacity is straightforward when discharging with a constant current until reaching the termination voltage. The formula to calculate the actual capacity of the battery is as follows:
Actual Capacity (Ct) = Discharge Current (I) × Discharge Time (t)
Where:
- Ct is the actual capacity of the battery in ampere-hours (Ah).
- I is the discharge current in amperes (A).
- t is the discharge time in hours (h).
To calculate the actual capacity, simply multiply the discharge current by the discharge time. This calculation provides the battery’s capacity in Ah under the specified discharge conditions.