Sulfide Stress Cracking (SSC) is a type of corrosion cracking that can occur in susceptible materials exposed to a sulfide-containing environment, typically in industries such as oil and gas production, refining, and chemical processing.
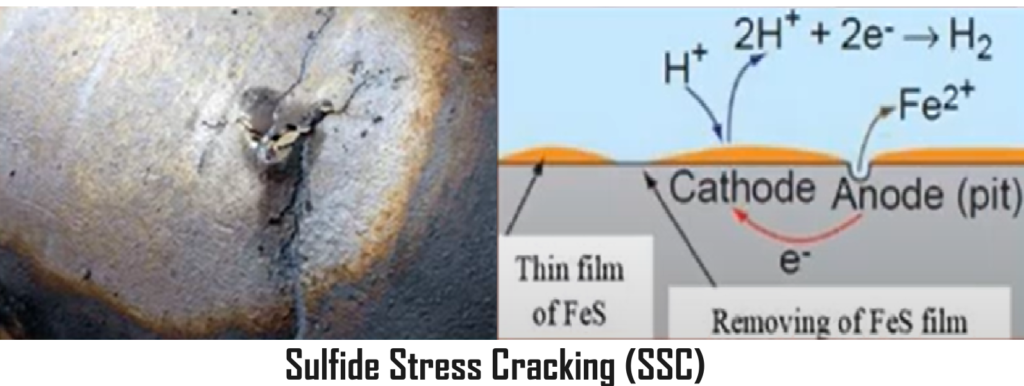
Sulfide Stress Corrosion Cracking.
Sulfide Stress cracking (SSC) is the trans‐granular cracking caused by absorbed atomic
hydrogen, at the locations of high stress and hardness in the weld and the HAZ. Zones of
high hardness are found in weld cover passes and attachment welds which are not
tempered (softened) by subsequent passes.
The hard areas of weldments, including both the weld deposit and heat affected zone which contain un‐tempered martensite and bainite microstructures. These microstructures possess hardness greater than 230 HB. Atomic hydrogen from the process stream is absorbed into these microstructures and causes cracking.
The crack once initiated propagates quickly through the localized hard zone, but slow down or stop when it enters a softer region (< 200 HB). However in presence of the stresses due to local deformation or cyclic stresses the SSC can propagate to the areas of hardness levels upto150 HB. SSC can be avoided by reducing the stresses and hardness by post weld heat treatment.
Sulfide stress cracking is also often grouped into the hydrogen embrittlement damages
discussed in Para 3.2 of this section. Further details on this damage mechanism can be
seen from API‐RP‐571 Para 5.1.2.3.1.d and Para 4.5.6.
Environment.
Sulfide Stress Cracking occurs in environments where hydrogen sulfide (H2S) gas is present, often in combination with water (aqueous environments). Hydrogen sulfide is highly toxic and corrosive, and when it comes into contact with certain metals, it can lead to the formation of hydrogen atoms, which can permeate into the metal structure, causing embrittlement.
Susceptible Materials.
Sulfide Stress Cracking is particularly problematic for high-strength carbon steels and low-alloy steels used in pipelines, well casings, and other equipment in the oil and gas industry. These materials are commonly exposed to H2S-containing environments during exploration, production, transportation, and refining processes.
Mechanism.
The mechanism of Sulfide Stress Cracking involves a combination of factors. Hydrogen sulfide gas dissolves in water, forming acidic solutions, which can accelerate the corrosion of metal surfaces. This corrosion process generates atomic hydrogen, which can penetrate into the metal structure, causing hydrogen embrittlement. The presence of tensile stresses, either residual or applied, further exacerbates the susceptibility to cracking.
Crack Formation.
Cracking in Sulfide Stress Cracking typically occurs along the grain boundaries of the metal, where hydrogen atoms accumulate, weakening the bonds between the grains. This can lead to the initiation and propagation of cracks, ultimately compromising the structural integrity of the material.
Prevention and Mitigation.
Preventing Sulfide Stress Cracking involves various measures, including selecting materials resistant to sulfide stress cracking, implementing corrosion control strategies such as coatings or inhibitors, controlling the concentration of H2S in the environment, and applying appropriate stress-relief treatments. Regular inspection and monitoring for signs of corrosion and cracking are also essential for early detection and maintenance.
In industries where H2S exposure is common, understanding and mitigating the risk of sulfide stress corrosion cracking are critical for ensuring the safety and reliability of infrastructure and equipment.