FTIR, which stands for Fourier Transform InfraRed, is the preferred method for conducting infrared spectroscopy. This analytical technique involves passing infrared (IR) radiation through a sample. During this process, some of the IR radiation is absorbed by the sample, while the rest is transmitted through it. The resulting spectrum provides insights into the molecular absorption and transmission patterns, essentially creating a unique molecular fingerprint for the sample. Much like human fingerprints, no two distinct molecular structures yield the same infrared spectrum. This characteristic makes infrared spectroscopy a valuable tool for various types of analyses.
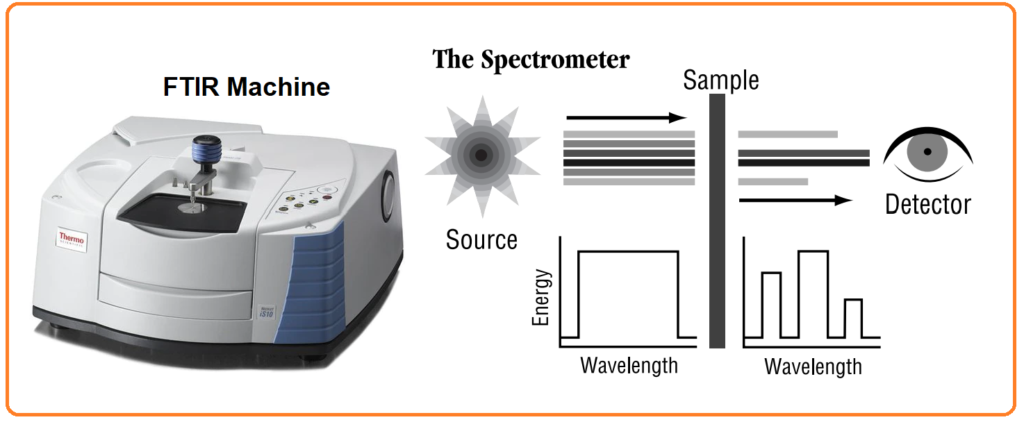
FTIR spectroscopy can provide the following information:
- Identification of Unknown Materials: It can help identify materials whose composition is not known, aiding in material characterization and classification.
- Quality and Consistency Determination: FTIR can assess the quality and uniformity of a sample, making it useful for quality control and ensuring product consistency.
- Quantification of Components: It can determine the quantity or concentration of individual components within a mixture, allowing for precise compositional analysis.
This article serves as an introduction to the fundamental principles of FTIR spectroscopy, explaining its underlying theory, used for, advantages, layout, diagram, working principle, sample process and operational mechanisms. Additionally, it discusses practical considerations related to the application of FTIR. Our aim is to provide you with a solid understanding of the significance and versatility of this powerful analytical technique.
What is Infrared Spectroscopy?
Infrared spectroscopy has been a cornerstone technique in material analysis laboratories for more than seven decades. It offers a powerful method for characterizing materials by generating an infrared spectrum, which serves as a distinctive fingerprint of a given sample. This spectrum exhibits absorption peaks that align with the frequencies of atomic bond vibrations within the material’s composition. Since each material consists of a unique combination of atoms, no two compounds yield precisely identical infrared spectra. As a result, infrared spectroscopy excels in positively identifying various materials, enabling qualitative analysis.
Moreover, the intensity of the peaks in the spectrum directly correlates with the quantity of the material present in the sample. Thanks to advanced software algorithms, infrared spectroscopy has evolved into an exceptional tool for quantitative analysis. This capability makes it an invaluable asset for accurately determining the concentration of substances in a sample.
Why FTIR is Developed?
Fourier Transform Infrared (FTIR) spectrometry emerged as a solution to address the limitations encountered with dispersive instruments, primarily the slow scanning process they employed. The key challenge was finding a method to measure all infrared frequencies simultaneously, rather than one by one. To tackle this issue, a clever solution was devised involving a simple optical device known as an interferometer.
The interferometer generates a unique type of signal that effectively encapsulates all infrared frequencies within it. What sets this technique apart is its remarkable speed – measurements can typically be made in just a matter of seconds, a substantial improvement compared to the several minutes required by traditional methods.
Working of FTIR:
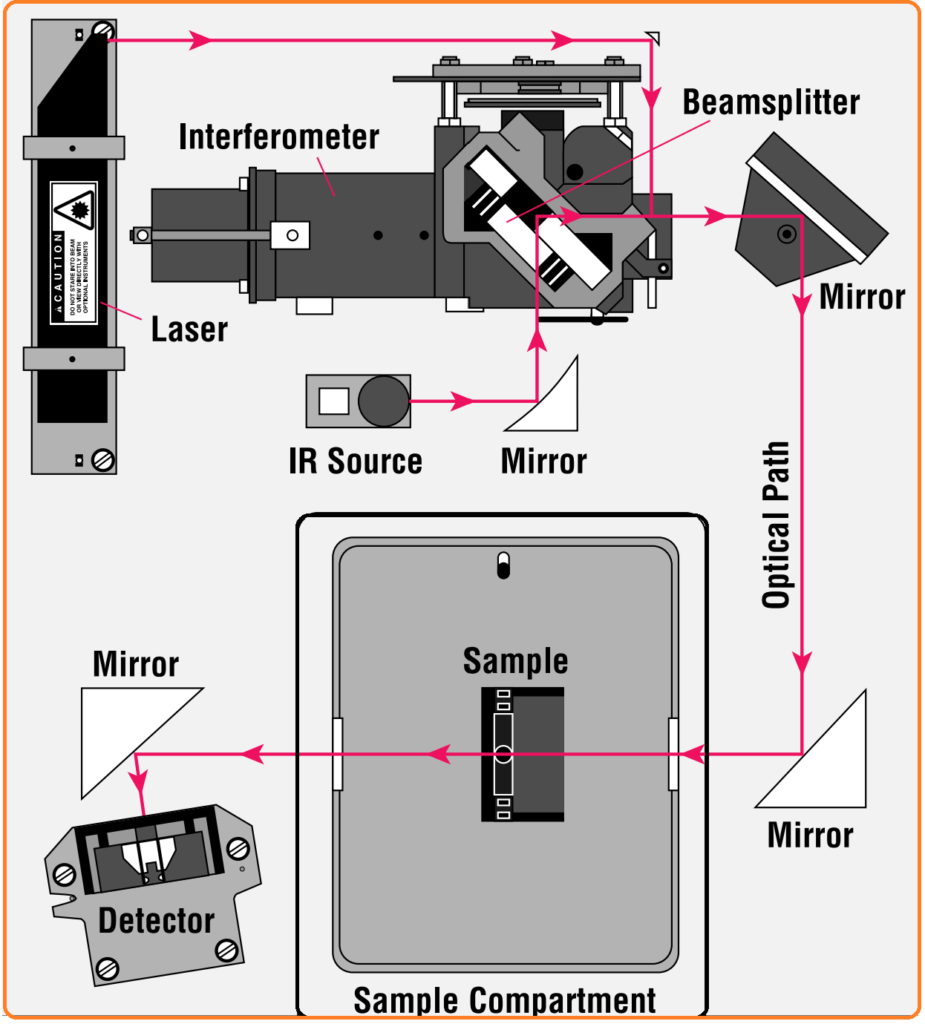
In most interferometers, an essential component is the beamsplitter, which divides the incoming infrared beam into two optical beams. One of these beams reflects off a stationary flat mirror, while the other reflects off another flat mirror attached to a mechanism that allows it to move a very short distance (usually a few millimeters) away from the beamsplitter. When these two beams reconvene at the beamsplitter, their paths have differed – one is fixed, and the other’s length changes as its mirror moves. This leads to the phenomenon of “interference,” giving rise to a signal known as an interferogram.
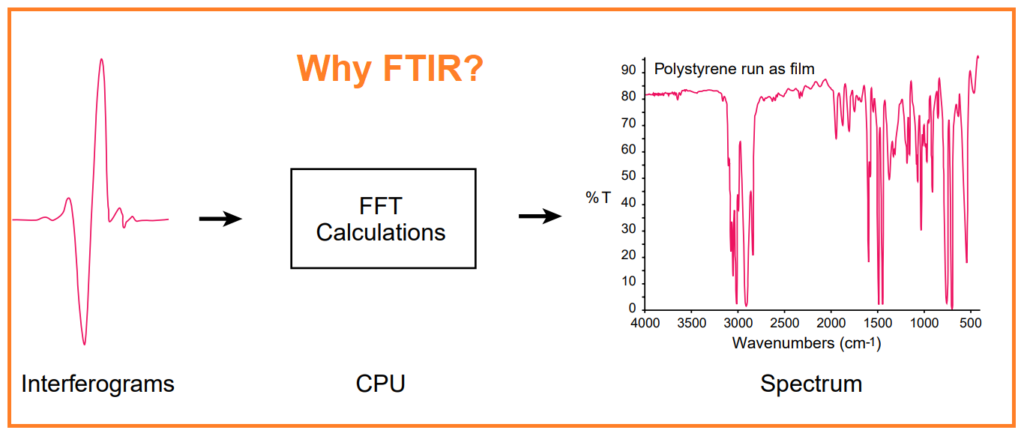
The interferogram possesses a distinctive property: each data point within it, determined by the moving mirror’s position, contains information about every infrared frequency originating from the source. Consequently, as the interferogram is measured, all frequencies are being simultaneously captured. This application of the interferometer translates into exceptionally rapid measurements.
However, since analysts require a frequency spectrum – essentially a plot displaying the intensity at each individual frequency – to make identifications, the measured interferogram signal cannot be directly interpreted. What’s needed is a method to “decode” these individual frequencies, and this is accomplished through a well-established mathematical technique called the Fourier transformation. The computer performs this transformation and subsequently presents users with the desired spectral information for analysis.
FTIR Sample Analysis Process
The standard instrumental process in Fourier Transform Infrared (FT-IR) spectrometry follows a series of key steps:
- The Source: Infrared energy is emitted from a radiant black-body source. This emitted beam is directed through an aperture, which functions to regulate the amount of energy delivered to the sample and subsequently to the detector.
- The Interferometer: The infrared beam enters the interferometer, where the pivotal “spectral encoding” occurs. The outcome of this process is the generation of the interferogram signal, which then exits the interferometer.
- The Sample: The infrared beam proceeds to the sample compartment, where it interacts with the sample. Depending on the type of analysis being conducted, the beam is either transmitted through the sample or reflected off its surface. It is within this stage that specific frequencies of energy are absorbed, uniquely characterizing the sample.
- The Detector: Subsequently, the beam reaches the detector, specially designed to measure the distinctive interferogram signal.
- The Computer: The acquired signal is digitized and transmitted to the computer, where the crucial Fourier transformation is performed. The final output is the infrared spectrum, which is then presented to the user for interpretation and any further analysis.
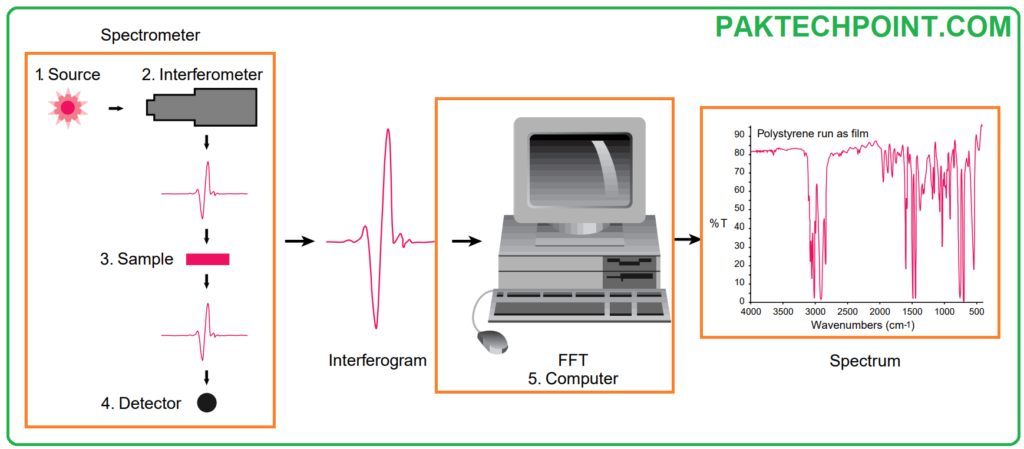
To establish a relative scale for absorption intensity, it is necessary to measure a background spectrum. Typically, this involves recording a measurement with no sample present in the beam. This background spectrum serves as a reference against which measurements with the sample are compared, enabling the calculation of “percent transmittance.” This method effectively eliminates instrumental characteristics from the spectrum, ensuring that all spectral features are exclusively attributed to the sample. A single background measurement can be applied to multiple sample measurements since it characterizes the instrument itself.
Spectrometer Layout Explanation:
FTIR Advantages:
FTIR spectroscopy offers several noteworthy advantages when compared to the dispersive technique:
- Speed: FTIR measures all frequencies simultaneously, resulting in measurements taking just seconds rather than minutes. This efficiency is often referred to as the Felgett Advantage.
- Sensitivity: FTIR significantly enhances sensitivity for various reasons. It employs highly sensitive detectors, boasts improved optical throughput (known as the Jacquinot Advantage), which leads to reduced noise levels, and enables the coaddition of multiple scans to reduce random measurement noise to the desired level (termed signal averaging).
- Mechanical Simplicity: FTIR instruments feature minimal moving parts, primarily limited to the moving mirror in the interferometer. This simplicity greatly reduces the likelihood of mechanical breakdown.
- Internal Calibration: FTIR instruments use a HeNe laser as an internal wavelength calibration standard, known as the Connes Advantage. As a result, these instruments are self-calibrating and do not require user calibration.
These advantages, among others, contribute to the precision and reliability of FTIR measurements. The heightened sensitivity enables the identification of even minute contaminants, making it a valuable tool for quality control and assurance tasks, including batch-to-batch comparisons against quality standards or the analysis of unidentified contaminants. Additionally, FTIR detectors’ sensitivity and accuracy, combined with a range of software algorithms, have significantly expanded the practical application of infrared spectroscopy in quantitative analysis. Developing and calibrating quantitative methods is simplified, allowing for their integration into routine analytical procedures. Consequently, Fourier Transform Infrared (FTIR) spectroscopy has introduced substantial practical benefits to infrared analysis, opening up possibilities for addressing complex challenges that were previously insurmountable with older technology. It has made the utilization of infrared analysis nearly boundless.
FTIR Used For
FT-IR (Fourier Transform Infrared) spectroscopy is a versatile analytical technique used in various fields for a wide range of applications. Some of the common applications of FT-IR include:
- Chemical Analysis: FT-IR is extensively used for the identification and characterization of chemical compounds. It can determine the functional groups present in a sample, aiding in the qualitative analysis of chemicals.
- Pharmaceuticals: In the pharmaceutical industry, FT-IR is employed for drug formulation, quality control, and the analysis of drug interactions. It helps ensure the consistency and quality of pharmaceutical products.
- Environmental Monitoring: FT-IR is used to analyze environmental samples, including air, water, and soil, to detect pollutants, contaminants, and toxins. It plays a crucial role in environmental monitoring and assessment.
- Materials Science: Researchers use FT-IR to study the composition, structure, and properties of various materials, such as polymers, plastics, composites, and coatings. It aids in material characterization and quality control.
- Food and Beverage Industry: FT-IR helps in food analysis, quality control, and safety assessment. It can identify ingredients, detect contaminants, and assess nutritional content.
- Forensics: Forensic scientists use FT-IR to analyze evidence, including drugs, fibers, paints, and trace materials. It assists in criminal investigations and evidence analysis.
- Pharmaceuticals: FT-IR is vital for drug formulation, quality control, and the analysis of drug interactions. It helps ensure the consistency and quality of pharmaceutical products.
- Medical Diagnostics: FT-IR can be used in medical research and diagnostics, such as identifying disease biomarkers, studying tissue samples, and analyzing bodily fluids.
- Art and Cultural Heritage Conservation: FT-IR is employed to analyze artworks, artifacts, and historical objects to understand their composition, deterioration, and restoration needs.
- Chemical Process Monitoring: In industrial settings, FT-IR is used for real-time monitoring of chemical processes. It ensures the efficiency and safety of chemical manufacturing.