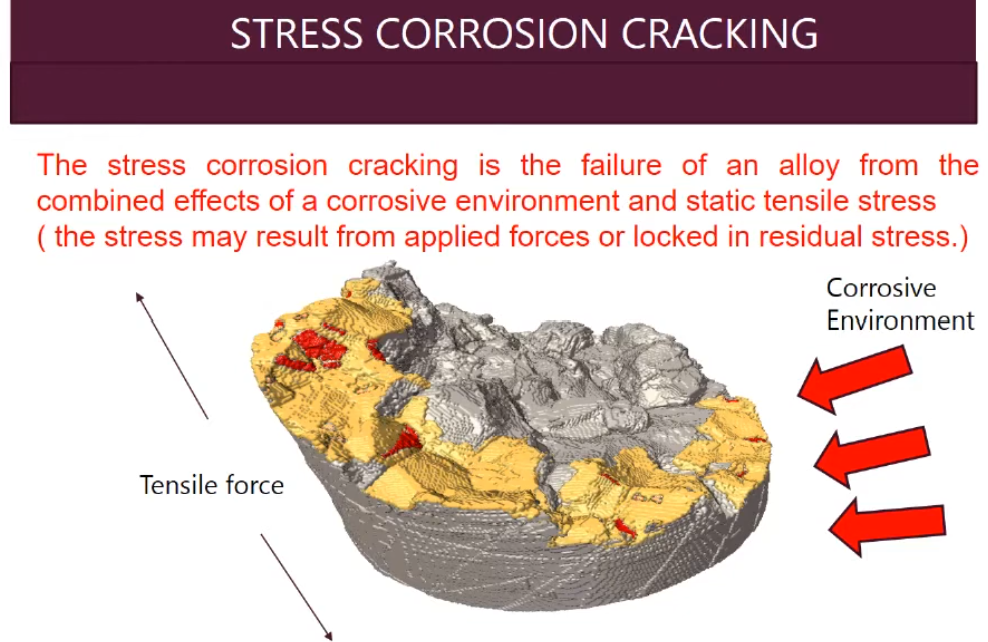
Stress Corrosion Cracking also known as Environmental Cracking. It is a group of damage mechanisms in which cracks are initiated and propagate under the simultaneous effect of a tensile stress and corrosion due to environment.
Stress Corrosion Cracking Explanation.
The tensile stresses originate from processes like cold forming, welding, heat treatment, machining and grinding, while corrosive environment is introduced by various process conditions. Because most of the surface of affected material remains un‐attacked, it is very difficult to detect or predict the failure.
Fine cracks develop and penetrating into the material at accelerated rate. These cracks can have an intergranular or a trans granular morphology. All of the SCC mechanisms can result in catastrophic failure with minimal overall material loss. Macroscopically, stress corrosion cracking fractures have a brittle appearance.
There are mainly two mechanisms of crack propagation in stress corrosion cracking. One is called as “Active Path Dissolution” in which crack propagates as the metal dissolves at the tip of the crack due to corrosion.
The second mechanism is “Hydrogen Embrittlement”, in which hydrogen gas produced by electrochemical process is absorbed into the metal and breaks cohesive structure of the metal. The main difference between two mechanisms is that Active Path Dissolution is intergranular SCC, while Hydrogen Embrittlement is trans granular form of stress corrosion cracking.
Active Path Dissolution Stress Corrosion Mechanisms.
There are various Stress corrosion cracking mechanisms which follow the inter‐granular active path dissolution techniques. The most prominent damage mechanisms in this group are given below.
Hydrogen Related damage (Wet H2S Damage).
Common material used in the manufacturing of the process equipment, especially the Carbon Steel and low alloy steel are susceptible to various types of Hydrogen related damages. These damages originate from Sour corrosion caused due the presence of wetH2S in process streams and cause materials to fail at stress levels well below their normalyield strength.
These damages occur when steel is exposed to a minimum of 50 ppm H2S and liquid water. Main course of all hydrogen related damage involves the migration of ionic hydrogen inside the material. The Hydrogen sulfide dissolved in water forms a weak acid generating high concentration of free H+ ions. These ions can easily migrate through the lattice of the steel and are absorbed in the grain boundaries of the in the locations of discontinuities like laminations.
The migrated ions than combine to form the hydrogen gas molecules H2, which being because of larger size cannot migrate out of the steel. As the pressure of the trapped gas increases within cavity, it tears the material lattice causing the cracks within the grain boundaries. This results in the reduction of the material strength, hence failure at stresses well below the established yield strength of the material. These damages appear in form of blistering and/or cracking. Following are some of the most common hydrogen damages found in plant and refinery process facilities.
Read Also following articles which are parts of stress corrosion cracking.
- Hydrogen Induced Cracking (HIC) and Hydrogen Blistering.
- What is Hydrogen Embrittlement?
- What is Amine Stress Corrosion Cracking?
- What is Caustic Embrittlement?
- What is Chloride Stress Corrosion Cracking (CI–SCC)?
- What is Sulfide Stress Cracking (SSC)?